Neutrophil transmigration mediated by the neutrophil-specific antigen CD177 is influenced by the endothelial S536N dimorphism of platelet endothelial cell adhesion molecule-1
- PMID: 20194726
- PMCID: PMC4154536
- DOI: 10.4049/jimmunol.0903136
Neutrophil transmigration mediated by the neutrophil-specific antigen CD177 is influenced by the endothelial S536N dimorphism of platelet endothelial cell adhesion molecule-1
Abstract
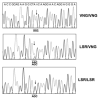
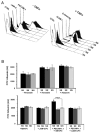
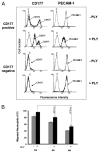
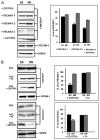
The human neutrophil-specific adhesion molecule CD177 (also known as the NB1 alloantigen) becomes upregulated on the cell surface in a number of inflammatory settings. We recently showed that CD177 functions as a novel heterophilic counterreceptor for the endothelial junctional protein PECAM-1 (CD31), an interaction that is mediated by membrane-proximal PECAM-1 IgD 6, which is known to harbor an S(536)N single nucleotide polymorphism of two major isoforms V(98)N(536)G(643) and L(98)S(536)R(643) and a yet-to-be-determined region on CD177. In vitro transendothelial migration experiments revealed that CD177(+) neutrophils migrated significantly faster through HUVECs expressing the LSR, compared with the VNG, allelic variant of PECAM-1 and that this correlated with the decreased ability of anti-PECAM-1 Ab of ITIM tyrosine phosphorylation in HUVECs expressing the LSR allelic variant relative to the VNG allelic variant. Moreover, engagement of PECAM-1 with rCD177-Fc (to mimic heterophilic CD177 binding) suppressed Ab-induced tyrosine phosphorylation to a greater extent in cells expressing the LSR isoform compared with the VNG isoform, with a corresponding increased higher level of beta-catenin phosphorylation. These data suggest that heterophilic PECAM-1/CD177 interactions affect the phosphorylation state of PECAM-1 and endothelial cell junctional integrity in such a way as to facilitate neutrophil transmigration in a previously unrecognized allele-specific manner.
Figures


Similar articles
-
The neutrophil-specific antigen CD177 is a counter-receptor for platelet endothelial cell adhesion molecule-1 (CD31).J Biol Chem. 2007 Aug 10;282(32):23603-12. doi: 10.1074/jbc.M701120200. Epub 2007 Jun 19. J Biol Chem. 2007. PMID: 17580308
-
Interaction between CD177 and platelet endothelial cell adhesion molecule-1 downregulates membrane-bound proteinase-3 (PR3) expression on neutrophils and attenuates neutrophil activation induced by PR3-ANCA.Arthritis Res Ther. 2018 Sep 20;20(1):213. doi: 10.1186/s13075-018-1710-0. Arthritis Res Ther. 2018. PMID: 30236159 Free PMC article.
-
Comparative evaluation of the role of the adhesion molecule CD177 in neutrophil interactions with platelets and endothelium.Eur J Haematol. 2012 Sep;89(3):236-44. doi: 10.1111/j.1600-0609.2012.01817.x. Epub 2012 Jul 10. Eur J Haematol. 2012. PMID: 22690867
-
Neutrophil-specific antigen HNA-2a, NB1 glycoprotein, and CD177.Curr Opin Hematol. 2007 Nov;14(6):688-93. doi: 10.1097/MOH.0b013e3282efed9e. Curr Opin Hematol. 2007. PMID: 17898576 Review.
-
Neutrophil-specific antigen HNA-2a (NB1, CD177): serology, biochemistry, and molecular biology.Vox Sang. 2002 Aug;83 Suppl 1:359-61. doi: 10.1111/j.1423-0410.2002.tb05334.x. Vox Sang. 2002. PMID: 12617169 Review. No abstract available.
Cited by
-
Asn563Ser polymorphism of CD31/PECAM-1 is associated with atherosclerotic cerebral infarction in a southern Han population.Neuropsychiatr Dis Treat. 2014 Dec 18;11:15-20. doi: 10.2147/NDT.S75065. eCollection 2015. Neuropsychiatr Dis Treat. 2014. PMID: 25565847 Free PMC article.
-
Airspaces-derived exosomes contain disease-relevant protein signatures in a mouse model of cystic fibrosis (CF)-like mucoinflammatory lung disease.Front Pharmacol. 2024 Sep 25;15:1460692. doi: 10.3389/fphar.2024.1460692. eCollection 2024. Front Pharmacol. 2024. PMID: 39386033 Free PMC article.
-
A Potent Leukocyte Transmigration Blocker: GT-73 Showed a Protective Effect against LPS-Induced ARDS in Mice.Molecules. 2021 Jul 29;26(15):4583. doi: 10.3390/molecules26154583. Molecules. 2021. PMID: 34361736 Free PMC article.
-
Meta-Analytic Comparison of Global RNA Transcriptomes of Acute and Chronic Myeloid Leukemia Cells Reveals Novel Gene Candidates Governing Myeloid Malignancies.Cancers (Basel). 2022 Sep 26;14(19):4681. doi: 10.3390/cancers14194681. Cancers (Basel). 2022. PMID: 36230605 Free PMC article.
-
Bone marrow transcriptome and epigenome profiles of equine common variable immunodeficiency patients unveil block of B lymphocyte differentiation.Clin Immunol. 2015 Oct;160(2):261-76. doi: 10.1016/j.clim.2015.05.005. Epub 2015 May 16. Clin Immunol. 2015. PMID: 25988861 Free PMC article.
References
-
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. - PubMed
-
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. - PubMed
-
- Rao RM, Yang L, Garcia-Cardena G, Luscinskas FW. Endothelial-dependent mechanisms of leukocyte recruitment to the vascular wall. Circ Res. 2007;101:234–247. - PubMed
-
- Muller WA, Randolph GJ. Migration of leukocytes across endothelium and beyond: molecules involved in the transmigration and fate of monocytes. J Leukoc Biol. 1999;66:698–704. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

