Abundant ribonucleotide incorporation into DNA by yeast replicative polymerases
- PMID: 20194773
- PMCID: PMC2841928
- DOI: 10.1073/pnas.0914857107
Abundant ribonucleotide incorporation into DNA by yeast replicative polymerases
Abstract
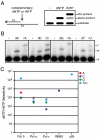
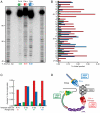
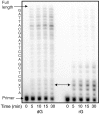
Measurements of nucleoside triphosphate levels in Saccharomyces cerevisiae reveal that the four rNTPs are in 36- to 190-fold molar excess over their corresponding dNTPs. During DNA synthesis in vitro using the physiological nucleoside triphosphate concentrations, yeast DNA polymerase epsilon, which is implicated in leading strand replication, incorporates one rNMP for every 1,250 dNMPs. Pol delta and Pol alpha, which conduct lagging strand replication, incorporate one rNMP for every 5,000 or 625 dNMPs, respectively. Discrimination against rNMP incorporation varies widely, in some cases by more than 100-fold, depending on the identity of the base and the template sequence context in which it is located. Given estimates of the amount of replication catalyzed by Pols alpha, delta, and epsilon, the results are consistent with the possibility that more than 10,000 rNMPs may be incorporated into the nuclear genome during each round of replication in yeast. Thus, rNMPs may be the most common noncanonical nucleotides introduced into the eukaryotic genome. Potential beneficial and negative consequences of abundant ribonucleotide incorporation into DNA are discussed, including the possibility that unrepaired rNMPs in DNA could be problematic because yeast DNA polymerase epsilon has difficulty bypassing a single rNMP present within a DNA template.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Kornberg A, Baker T. DNA replication. 2nd Ed. New York: Freeman; 1992.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

