Full-length extracellular region of the var2CSA variant of PfEMP1 is required for specific, high-affinity binding to CSA
- PMID: 20194779
- PMCID: PMC2841952
- DOI: 10.1073/pnas.1000951107
Full-length extracellular region of the var2CSA variant of PfEMP1 is required for specific, high-affinity binding to CSA
Abstract
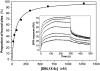
Pregnancy-associated malaria (PAM) is a serious consequence of sequestration of Plasmodium falciparum-parasitized erythrocytes (PE) in the placenta through adhesion to chondroitin sulfate A (CSA) present on placental proteoglycans. Recent work implicates var2CSA, a member of the PfEMP1 family, as the mediator of placental sequestration and as a key target for PAM vaccine development. Var2CSA is a 350 kDa transmembrane protein, whose extracellular region includes six Duffy-binding-like (DBL) domains. Due to its size and high cysteine content, the full-length var2CSA extracellular region has not hitherto been expressed in heterologous systems, thus limiting investigations to individual recombinant domains. Here we report for the first time the expression of the full-length var2CSA extracellular region (domains DBL1X to DBL6epsilon) from the 3D7 parasite strain using the human embryonic kidney 293 cell line. We show that the recombinant extracellular var2CSA region is correctly folded and that, unlike the individual DBL domains, it binds with high affinity and specificity to CSA (K(D) = 61 nM) and efficiently inhibits PE from binding to CSA. Structural characterization by analytical ultracentrifugation and small-angle x-ray scattering reveals a compact organization of the full-length protein, most likely governed by specific interdomain interactions, rather than an extended structure. Collectively, these data suggest that a high-affinity, CSA-specific binding site is formed by the higher-order structure of the var2CSA extracellular region. These results have important consequences for the development of an effective vaccine and therapeutic inhibitors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


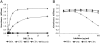

Similar articles
-
Structural and functional insight into how the Plasmodium falciparum VAR2CSA protein mediates binding to chondroitin sulfate A in placental malaria.J Biol Chem. 2012 Jul 6;287(28):23332-45. doi: 10.1074/jbc.M112.348839. Epub 2012 May 8. J Biol Chem. 2012. PMID: 22570492 Free PMC article.
-
Chondroitin sulphate A (CSA)-binding of single recombinant Duffy-binding-like domains is not restricted to Plasmodium falciparum Erythrocyte Membrane Protein 1 expressed by CSA-binding parasites.Int J Parasitol. 2009 Sep;39(11):1195-204. doi: 10.1016/j.ijpara.2009.02.022. Epub 2009 Mar 24. Int J Parasitol. 2009. PMID: 19324047
-
Structural insights into chondroitin sulphate A binding Duffy-binding-like domains from Plasmodium falciparum: implications for intervention strategies against placental malaria.Malar J. 2009 Apr 17;8:67. doi: 10.1186/1475-2875-8-67. Malar J. 2009. PMID: 19374733 Free PMC article.
-
Structural insights into chondroitin sulfate binding in pregnancy-associated malaria.Biochem Soc Trans. 2010 Oct;38(5):1337-41. doi: 10.1042/BST0381337. Biochem Soc Trans. 2010. PMID: 20863310 Review.
-
Designing a VAR2CSA-based vaccine to prevent placental malaria.Vaccine. 2015 Dec 22;33(52):7483-8. doi: 10.1016/j.vaccine.2015.10.011. Epub 2015 Nov 26. Vaccine. 2015. PMID: 26469717 Free PMC article. Review.
Cited by
-
Adhesion of Plasmodium falciparum infected erythrocytes in ex vivo perfused placental tissue: a novel model of placental malaria.Malar J. 2016 May 26;15(1):292. doi: 10.1186/s12936-016-1342-2. Malar J. 2016. PMID: 27230523 Free PMC article.
-
Multiple Plasmodium falciparum Erythrocyte Membrane Protein 1 Variants per Genome Can Bind IgM via Its Fc Fragment Fcμ.Infect Immun. 2015 Oct;83(10):3972-81. doi: 10.1128/IAI.00337-15. Epub 2015 Jul 27. Infect Immun. 2015. PMID: 26216422 Free PMC article.
-
Structural and functional insight into how the Plasmodium falciparum VAR2CSA protein mediates binding to chondroitin sulfate A in placental malaria.J Biol Chem. 2012 Jul 6;287(28):23332-45. doi: 10.1074/jbc.M112.348839. Epub 2012 May 8. J Biol Chem. 2012. PMID: 22570492 Free PMC article.
-
Sequence polymorphism, segmental recombination and toggling amino acid residues within the DBL3X domain of the VAR2CSA placental malaria antigen.PLoS One. 2012;7(2):e31565. doi: 10.1371/journal.pone.0031565. Epub 2012 Feb 9. PLoS One. 2012. PMID: 22347496 Free PMC article.
-
Impact of Hemoglobin S Trait on Cell Surface Antibody Recognition of Plasmodium falciparum-Infected Erythrocytes in Pregnancy-Associated Malaria.Open Forum Infect Dis. 2019 Mar 29;6(4):ofz156. doi: 10.1093/ofid/ofz156. eCollection 2019 Apr. Open Forum Infect Dis. 2019. PMID: 31041352 Free PMC article.
References
-
- Brabin BJ, et al. The sick placenta-the role of malaria. Placenta. 2004;25(5):359–378. - PubMed
-
- Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996;272(5267):1502–1504. - PubMed
-
- Alkhalil A, Achur RN, Valiyaveettil M, Ockenhouse CF, Gowda DC. Structural requirements for the adherence of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate proteoglycans of human placenta. J Biol Chem. 2000;275(51):40357–40364. - PubMed
-
- Salanti A, et al. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol Microbiol. 2003;49(1):179–191. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

