Induction of tissue factor by urokinase in lung epithelial cells and in the lungs
- PMID: 20194819
- PMCID: PMC2894411
- DOI: 10.1164/rccm.200901-0015OC
Induction of tissue factor by urokinase in lung epithelial cells and in the lungs
Abstract
Rationale: Urokinase-type plasminogen activator (uPA) regulates extracellular proteolysis in lung injury and repair. Although alveolar expression of uPA increases, procoagulant activity predominates.
Objectives: This study was designed to investigate whether uPA alters the expression of tissue factor (TF), the major initiator of the coagulation cascade, in lung epithelial cells (ECs).
Methods: Bronchial, primary airway ECs and C57B6 wild-type, uPA-deficient (uPA(-/-)) mice were exposed to phosphate-buffered saline, uPA, or LPS. Immunohistochemistry, protein, cellular, and molecular techniques were used to assess TF expression and activity.
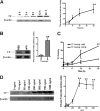
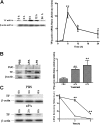
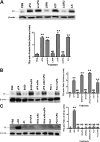
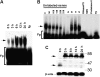
Measurements and main results: uPA enhanced TF mRNA and protein expression, and TF-dependent coagulation in lung ECs. uPA-induced expression of TF involves both increased synthesis and enhanced stabilization of TF mRNA. uPA catalytic activity had little effect on induction of TF. By contrast, deletion of the uPA receptor binding growth factor domain from uPA markedly attenuated the induction of TF, suggesting that uPA receptor binding is sufficient for TF induction. Lung tissues of uPA-deficient mice expressed less TF protein and mRNA compared with wild-type mice. In addition, intratracheal instillation of mouse uPA increased TF mRNA and protein expression and accelerated coagulation in lung tissues. uPA(-/-) mice exposed to LPS failed to induce TF.
Conclusions: uPA increased TF expression and TF-dependent coagulation in the lungs of mice. We hypothesize that uPA-mediated induction of TF occurs in lung ECs to promote increased fibrin deposition in the airways during acute lung injury.
Figures




Similar articles
-
Induction of plasminogen activator inhibitor-1 by urokinase in lung epithelial cells.J Biol Chem. 2003 May 16;278(20):18124-31. doi: 10.1074/jbc.M207445200. Epub 2003 Mar 17. J Biol Chem. 2003. PMID: 12642587
-
Coagulation, fibrinolysis, and fibrin deposition in acute lung injury.Crit Care Med. 2003 Apr;31(4 Suppl):S213-20. doi: 10.1097/01.CCM.0000057846.21303.AB. Crit Care Med. 2003. PMID: 12682443 Review.
-
Regulation of lung injury and fibrosis by p53-mediated changes in urokinase and plasminogen activator inhibitor-1.Am J Pathol. 2013 Jul;183(1):131-43. doi: 10.1016/j.ajpath.2013.03.022. Epub 2013 May 8. Am J Pathol. 2013. PMID: 23665346 Free PMC article.
-
Endothelium and disordered fibrin turnover in the injured lung: newly recognized pathways.Crit Care Med. 2002 May;30(5 Suppl):S274-80. doi: 10.1097/00003246-200205001-00017. Crit Care Med. 2002. PMID: 12004248 Review.
-
Post-transcriptional regulation of urokinase-type plasminogen activator receptor expression in lipopolysaccharide-induced acute lung injury.Am J Respir Crit Care Med. 2009 Feb 15;179(4):288-98. doi: 10.1164/rccm.200712-1787OC. Epub 2008 Nov 21. Am J Respir Crit Care Med. 2009. PMID: 19029002 Free PMC article.
Cited by
-
Regulation of airway and alveolar epithelial cell apoptosis by p53-Induced plasminogen activator inhibitor-1 during cigarette smoke exposure injury.Am J Respir Cell Mol Biol. 2012 Oct;47(4):474-83. doi: 10.1165/rcmb.2011-0390OC. Epub 2012 May 16. Am J Respir Cell Mol Biol. 2012. PMID: 22592924 Free PMC article.
-
Lung Epithelial Cell Transcriptional Regulation as a Factor in COVID-19-associated Coagulopathies.Am J Respir Cell Mol Biol. 2021 Jun;64(6):687-697. doi: 10.1165/rcmb.2020-0453OC. Am J Respir Cell Mol Biol. 2021. PMID: 33740387 Free PMC article.
-
Update in acute lung injury and critical care 2010.Am J Respir Crit Care Med. 2011 May 1;183(9):1147-52. doi: 10.1164/rccm.201102-0327UP. Am J Respir Crit Care Med. 2011. PMID: 21531954 Free PMC article. Review. No abstract available.
-
Unique transcriptional changes in coagulation cascade genes in SARS-CoV-2-infected lung epithelial cells: A potential factor in COVID-19 coagulopathies.bioRxiv [Preprint]. 2020 Jul 7:2020.07.06.182972. doi: 10.1101/2020.07.06.182972. bioRxiv. 2020. PMID: 32676594 Free PMC article. Preprint.
-
The role of mononuclear cell tissue factor and inflammatory cytokines in patients with chronic thromboembolic pulmonary hypertension.J Thromb Thrombolysis. 2016 Jul;42(1):38-45. doi: 10.1007/s11239-015-1323-2. J Thromb Thrombolysis. 2016. PMID: 26667361 Free PMC article.
References
-
- Abraham E, Gyetko MR, Kuhn K, Arcaroli J, Strassheim D, Park JS, Shetty S, Idell S. Urokinase-type plasminogen activator potentiates lipopolysaccharide-induced neutrophil activation. J Immunol 2003;170:5644–5651. - PubMed
-
- Hattori N, Sisson TH, Xu Y, Simon RH. Upregulation of fibrinolysis by adenovirus-mediated transfer of urokinase-type plasminogen activator genes to lung cells in vitro and in vivo. Hum Gene Ther 1999;10:215–222. - PubMed
-
- Chua F, Sly PD, Laurent GJ. Pediatric lung disease: from proteinases to pulmonary fibrosis. Pediatr Pulmonol 2005;39:392–401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

