Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia
- PMID: 20194866
- PMCID: PMC4979101
- DOI: 10.1200/JCO.2009.25.3187
Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia
Erratum in
- J Clin Oncol. 2010 Aug 1;28(22):3670
Abstract
Purpose: New treatments are needed for patients with fludarabine- and alemtuzumab-refractory (FA-ref) chronic lymphocytic leukemia (CLL) or patients with fludarabine-refractory CLL with bulky (> 5 cm) lymphadenopathy (BF-ref) who are less suitable for alemtuzumab treatment; these groups have poor outcomes with available salvage regimens. Ofatumumab (HuMax-CD20) is a human monoclonal antibody targeting a distinct small-loop epitope on the CD20 molecule. We conducted an international clinical study to evaluate the efficacy and safety of ofatumumab in patients with FA-ref and BF-ref CLL.
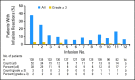
Patients and methods: Patients received eight weekly infusions of ofatumumab followed by four monthly infusions during a 24-week period (dose 1 = 300 mg; doses 2 to 12 = 2,000 mg); response by an independent review committee (1996 National Cancer Institute Working Group criteria) was assessed every 4 weeks until week 24 and then every 3 months until month 24.
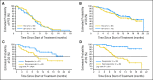
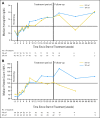
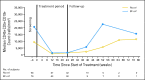
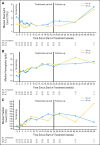
Results: This planned interim analysis included 138 treated patients with FA-ref (n = 59) and BF-ref (n = 79) CLL. The overall response rates (primary end point) were 58% [corrected] and 47% in the FA-ref and BF-ref groups, respectively. Complete resolution of constitutional symptoms and improved performance status occurred in 57% and 48% of patients, respectively. Median progression-free survival and overall survival times were 5.7 and 13.7 months in the FA-ref group, respectively, and 5.9 and 15.4 months in the BF-ref group, respectively. The most common adverse events during treatment were infusion reactions and infections, which were primarily grade 1 or 2 events. Hematologic events during treatment included anemia and neutropenia.
Conclusion: Ofatumumab is an active, well-tolerated treatment providing clear clinical improvements for fludarabine-refractory patients with very poor-prognosis CLL.
Trial registration: ClinicalTrials.gov NCT00349349.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
References
-
- Eichhorst BF, Busch R, Hopfinger G, et al. Fludarabine plus cyclophosphamide versus fludarabine alone in first-line therapy of younger patients with chronic lymphocytic leukemia. Blood. 2006;107:885–891. - PubMed
-
- Keating MJ, O'Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4079–4088. - PubMed
-
- Catovsky D, Richards S, Matutes E, et al. Assessment of fludarabine plus cyclophosphamide for patients with chronic lymphocytic leukaemia (the LRF CLL4 Trial): A randomised controlled trial. Lancet. 2007;370:230–239. - PubMed
-
- Flinn IW, Neuberg DS, Grever MR, et al. Phase III trial of fludarabine plus cyclophosphamide compared with fludarabine for patients with previously untreated chronic lymphocytic leukemia: US Intergroup Trial E2997. J Clin Oncol. 2007;25:793–798. - PubMed
-
- Wierda W, O'Brien S, Wen S, et al. Chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab for relapsed and refractory chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4070–4078. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

