Transformation/transcription domain-associated protein (TRRAP)-mediated regulation of Wee1
- PMID: 20194963
- PMCID: PMC2870978
- DOI: 10.1534/genetics.110.114769
Transformation/transcription domain-associated protein (TRRAP)-mediated regulation of Wee1
Abstract
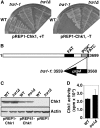
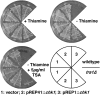
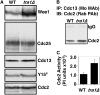
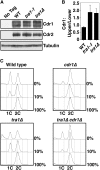
The G2 DNA damage checkpoint inhibits Cdc2 and mitotic entry through the dual regulation of Wee1 and Cdc25 by the Chk1 effector kinase. Upregulation of Chk1 by mutation or overexpression bypasses the requirement for upstream regulators or DNA damage to promote a G2 cell cycle arrest. We screened in fission yeast for mutations that rendered cells resistant to overexpressed chk1(+). We identified a mutation in tra1, which encodes one of two homologs of transformation/transcription domain-associated protein (TRRAP), an ATM/R-related pseudokinase that scaffolds several histone acetyltransferase (HAT) complexes. Inhibition of histone deacetylases reverts the resistance to overexpressed chk1(+), suggesting this phenotype is due to a HAT activity, although expression of checkpoint and cell cycle genes is not greatly affected. Cells with mutant or deleted tra1 activate Chk1 normally and are checkpoint proficient. However, these cells are semi-wee even when overexpressing chk1(+) and accumulate inactive Wee1 protein. The changed division response (Cdr) kinases Cdr1 and Cdr2 are negative regulators of Wee1, and we show that they are required for the Tra1-dependent alterations to Wee1 function. This identifies Tra1 as another component controlling the timing of entry into mitosis via Cdc2 activation.
Figures



Similar articles
-
Antagonism of Chk1 signaling in the G2 DNA damage checkpoint by dominant alleles of Cdr1.Genetics. 2006 Sep;174(1):113-23. doi: 10.1534/genetics.106.060970. Epub 2006 Jul 2. Genetics. 2006. PMID: 16816416 Free PMC article.
-
Cdc25 mitotic inducer targeted by chk1 DNA damage checkpoint kinase.Science. 1997 Sep 5;277(5331):1495-7. doi: 10.1126/science.277.5331.1495. Science. 1997. PMID: 9278510
-
The protein kinase Cdr2, related to Nim1/Cdr1 mitotic inducer, regulates the onset of mitosis in fission yeast.Mol Biol Cell. 1998 Dec;9(12):3321-34. doi: 10.1091/mbc.9.12.3321. Mol Biol Cell. 1998. PMID: 9843572 Free PMC article.
-
Reversible tyrosine phosphorylation and cell cycle control.Semin Cell Biol. 1993 Dec;4(6):433-42. doi: 10.1006/scel.1993.1051. Semin Cell Biol. 1993. PMID: 8305682 Review.
-
Regulation of G2/M Transition by Inhibition of WEE1 and PKMYT1 Kinases.Molecules. 2017 Nov 23;22(12):2045. doi: 10.3390/molecules22122045. Molecules. 2017. PMID: 29168755 Free PMC article. Review.
Cited by
-
Connecting chromatin modifying factors to DNA damage response.Int J Mol Sci. 2013 Jan 24;14(2):2355-69. doi: 10.3390/ijms14022355. Int J Mol Sci. 2013. PMID: 23348929 Free PMC article.
-
The SAGA histone acetyltransferase complex regulates leucine uptake through the Agp3 permease in fission yeast.J Biol Chem. 2012 Nov 2;287(45):38158-67. doi: 10.1074/jbc.M112.411165. Epub 2012 Sep 18. J Biol Chem. 2012. PMID: 22992726 Free PMC article.
-
SAGA function in tissue-specific gene expression.Trends Cell Biol. 2012 Apr;22(4):177-84. doi: 10.1016/j.tcb.2011.11.005. Epub 2011 Dec 21. Trends Cell Biol. 2012. PMID: 22196215 Free PMC article.
-
Deep sequencing of gastric carcinoma reveals somatic mutations relevant to personalized medicine.J Transl Med. 2011 Jul 25;9:119. doi: 10.1186/1479-5876-9-119. J Transl Med. 2011. PMID: 21781349 Free PMC article.
-
An insight into understanding the coupling between homologous recombination mediated DNA repair and chromatin remodeling mechanisms in plant genome: an update.Cell Cycle. 2021 Sep;20(18):1760-1784. doi: 10.1080/15384101.2021.1966584. Epub 2021 Aug 26. Cell Cycle. 2021. PMID: 34437813 Free PMC article. Review.
References
-
- Alfa, C. E., R. Booher, D. Beach and J. S. Hyams, 1989. Fission yeast cyclin: subcellular localisation and cell cycle regulation. J Cell Sci Suppl 12 9–19. - PubMed
-
- Basi, G., E. Schmid and K. Maundrell, 1993. TATA box mutations in the Schizosaccharomyces pombe nmt1 promoter affect transcription efficiency but not the transcription start point or thiamine repressibility. Gene 123 131–136. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

