A comprehensive resource of interacting protein regions for refining human transcription factor networks
- PMID: 20195357
- PMCID: PMC2827538
- DOI: 10.1371/journal.pone.0009289
A comprehensive resource of interacting protein regions for refining human transcription factor networks
Abstract
Large-scale data sets of protein-protein interactions (PPIs) are a valuable resource for mapping and analysis of the topological and dynamic features of interactome networks. The currently available large-scale PPI data sets only contain information on interaction partners. The data presented in this study also include the sequences involved in the interactions (i.e., the interacting regions, IRs) suggested to correspond to functional and structural domains. Here we present the first large-scale IR data set obtained using mRNA display for 50 human transcription factors (TFs), including 12 transcription-related proteins. The core data set (966 IRs; 943 PPIs) displays a verification rate of 70%. Analysis of the IR data set revealed the existence of IRs that interact with multiple partners. Furthermore, these IRs were preferentially associated with intrinsic disorder. This finding supports the hypothesis that intrinsically disordered regions play a major role in the dynamics and diversity of TF networks through their ability to structurally adapt to and bind with multiple partners. Accordingly, this domain-based interaction resource represents an important step in refining protein interactions and networks at the domain level and in associating network analysis with biological structure and function.
Conflict of interest statement
Figures

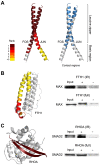
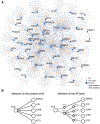


References
-
- Jeong H, Mason SP, Barabasi AL, Oltvai ZN. Lethality and centrality in protein networks. Nature. 2001;411:41–42. - PubMed
-
- Han JD, Bertin N, Hao T, Goldberg DS, Berriz GF, et al. Evidence for dynamically organized modularity in the yeast protein-protein interaction network. Nature. 2004;430:88–93. - PubMed
-
- Uetz P, Giot L, Cagney G, Mansfield TA, Judson RS, et al. A comprehensive analysis of protein-protein interactions in Saccharomyces cerevisiae. Nature. 2000;403:623–627. - PubMed
-
- Gavin AC, Bosche M, Krause R, Grandi P, Marzioch M, et al. Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature. 2002;415:141–147. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

