Protective effect of human amniotic fluid stem cells in an immunodeficient mouse model of acute tubular necrosis
- PMID: 20195358
- PMCID: PMC2827539
- DOI: 10.1371/journal.pone.0009357
Protective effect of human amniotic fluid stem cells in an immunodeficient mouse model of acute tubular necrosis
Abstract
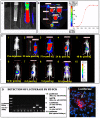
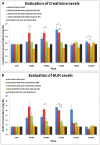
Acute Tubular Necrosis (ATN) causes severe damage to the kidney epithelial tubular cells and is often associated with severe renal dysfunction. Stem-cell based therapies may provide alternative approaches to treating of ATN. We have previously shown that clonal c-kit(pos) stem cells, derived from human amniotic fluid (hAFSC) can be induced to a renal fate in an ex-vivo system. Herein, we show for the first time the successful therapeutic application of hAFSC in a mouse model with glycerol-induced rhabdomyolysis and ATN. When injected into the damaged kidney, luciferase-labeled hAFSC can be tracked using bioluminescence. Moreover, we show that hAFSC provide a protective effect, ameliorating ATN in the acute injury phase as reflected by decreased creatinine and BUN blood levels and by a decrease in the number of damaged tubules and apoptosis therein, as well as by promoting proliferation of tubular epithelial cells. We show significant immunomodulatory effects of hAFSC, over the course of ATN. We therefore speculate that AFSC could represent a novel source of stem cells that may function to modulate the kidney immune milieu in renal failure caused by ATN.
Conflict of interest statement
Figures






Similar articles
-
Amniotic fluid stem cells ameliorate cisplatin-induced acute renal failure through induction of autophagy and inhibition of apoptosis.Stem Cell Res Ther. 2019 Dec 4;10(1):370. doi: 10.1186/s13287-019-1476-6. Stem Cell Res Ther. 2019. PMID: 31801607 Free PMC article.
-
Bmi-1 plays a critical role in the protection from acute tubular necrosis by mobilizing renal stem/progenitor cells.Biochem Biophys Res Commun. 2017 Jan 22;482(4):742-749. doi: 10.1016/j.bbrc.2016.11.105. Epub 2016 Nov 18. Biochem Biophys Res Commun. 2017. PMID: 27871857
-
Stem cells derived from human amniotic fluid contribute to acute kidney injury recovery.Am J Pathol. 2010 Oct;177(4):2011-21. doi: 10.2353/ajpath.2010.091245. Epub 2010 Aug 19. Am J Pathol. 2010. PMID: 20724594 Free PMC article.
-
Primary acute renal failure ("acute tubular necrosis") in the transplanted kidney: morphology and pathogenesis.Medicine (Baltimore). 1989 May;68(3):173-87. doi: 10.1097/00005792-198905000-00005. Medicine (Baltimore). 1989. PMID: 2654537 Review.
-
Acute renal failure. II. Experimental models of acute renal failure: imperfect but indispensable.Am J Physiol Renal Physiol. 2000 Jan;278(1):F1-F12. doi: 10.1152/ajprenal.2000.278.1.F1. Am J Physiol Renal Physiol. 2000. PMID: 10644651 Review.
Cited by
-
Pdx1 and controlled culture conditions induced differentiation of human amniotic fluid-derived stem cells to insulin-producing clusters.J Tissue Eng Regen Med. 2015 May;9(5):540-9. doi: 10.1002/term.1631. Epub 2012 Nov 13. J Tissue Eng Regen Med. 2015. PMID: 23147868 Free PMC article.
-
Regenerating a kidney in a lymph node.Pediatr Nephrol. 2016 Oct;31(10):1553-60. doi: 10.1007/s00467-015-3296-y. Epub 2015 Dec 21. Pediatr Nephrol. 2016. PMID: 26686504 Free PMC article. Review.
-
Therapeutic Potential of Stem Cells from Human Exfoliated Deciduous Teeth in Models of Acute Kidney Injury.PLoS One. 2015 Oct 28;10(10):e0140121. doi: 10.1371/journal.pone.0140121. eCollection 2015. PLoS One. 2015. PMID: 26509261 Free PMC article.
-
Cardiomyogenic Differentiation of Human Dental Follicle-derived Stem Cells by Suberoylanilide Hydroxamic Acid and Their In Vivo Homing Property.Int J Med Sci. 2016 Oct 18;13(11):841-852. doi: 10.7150/ijms.16573. eCollection 2016. Int J Med Sci. 2016. PMID: 27877076 Free PMC article.
-
Amniotic Fluid Derived Stem Cells with a Renal Progenitor Phenotype Inhibit Interstitial Fibrosis in Renal Ischemia and Reperfusion Injury in Rats.PLoS One. 2015 Aug 21;10(8):e0136145. doi: 10.1371/journal.pone.0136145. eCollection 2015. PLoS One. 2015. PMID: 26295710 Free PMC article.
References
-
- National Kidney and Urologic Diseases Information Clearinghouse. Kidney and Urologic Diseases Statistics for the United States. Available: http://kidney.niddk.nih.gov/kudiseases/pubs/kustats/index.htm#kpClearing....
-
- Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med. 1996;334(22):1448–1460. - PubMed
-
- Yokoo T, Fukui A, Ohashi T, Miyazaki Y, Utsunomiya Y, et al. Xenobiotic kidney organogenesis from human mesenchymal stem cells using a growing rodent embryo. J Am Soc Nephrol. 2006;17(4):1026–1034. - PubMed
-
- Herrera MB, Bussolati B, Bruno S, Fonsato V, Romanazzi GM, et al. Mesenchymal stem cells contribute to the renal repair of acute tubular epithelial injury. Int J Mol Med. 2004;14(6):1035–1041. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

