Oral biofilm architecture on natural teeth
- PMID: 20195365
- PMCID: PMC2827546
- DOI: 10.1371/journal.pone.0009321
Oral biofilm architecture on natural teeth
Abstract
Periodontitis and caries are infectious diseases of the oral cavity in which oral biofilms play a causative role. Moreover, oral biofilms are widely studied as model systems for bacterial adhesion, biofilm development, and biofilm resistance to antibiotics, due to their widespread presence and accessibility. Despite descriptions of initial plaque formation on the tooth surface, studies on mature plaque and plaque structure below the gum are limited to landmark studies from the 1970s, without appreciating the breadth of microbial diversity in the plaque. We used fluorescent in situ hybridization to localize in vivo the most abundant species from different phyla and species associated with periodontitis on seven embedded teeth obtained from four different subjects. The data showed convincingly the dominance of Actinomyces sp., Tannerella forsythia, Fusobacterium nucleatum, Spirochaetes, and Synergistetes in subgingival plaque. The latter proved to be new with a possibly important role in host-pathogen interaction due to its localization in close proximity to immune cells. The present study identified for the first time in vivo that Lactobacillus sp. are the central cells of bacterial aggregates in subgingival plaque, and that Streptococcus sp. and the yeast Candida albicans form corncob structures in supragingival plaque. Finally, periodontal pathogens colonize already formed biofilms and form microcolonies therein. These in vivo observations on oral biofilms provide a clear vision on biofilm architecture and the spatial distribution of predominant species.
Conflict of interest statement
Figures


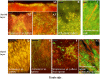
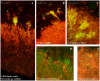

References
-
- Wood SR, Kirkham J, Marsh PD, Shore RC, Nattress B, et al. Architecture Of Intact Natural Human Plaque Biofilms Studied By Confocal Laser Scanning Microscopy. J Dent Res. 2000;79:21–27. - PubMed
-
- Socransky SS, Haffajee AD. Dental Biofilms: Difficult Therapeutic Targets. Periodontol 2000. 2002;28:12–55. - PubMed
-
- Reese S, Guggenheim B. A Novel TEM Contrasting Technique For Extracellular Polysaccharides In In Vitro Biofilms. Microsc Res Tech. 2007;70:816–822. - PubMed
-
- Bos R, Van Der Mei HC, Busscher HJ. Physico-Chemistry Of Initial Microbial Adhesive Interactions–Its Mechanisms And Methods For Study. FEMS Microbiol Rev. 1999;23:179–230. - PubMed
-
- Busscher HJ, Van Der Mei HC. Physico-Chemical Interactions In Initial Microbial Adhesion And Relevance For Biofilm Formation. Adv Dent Res. 1997;11:24–32. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

