Inhibition of in vitro fertilizing capacity of cryopreserved mouse sperm by factors released by damaged sperm, and stimulation by glutathione
- PMID: 20195370
- PMCID: PMC2827551
- DOI: 10.1371/journal.pone.0009387
Inhibition of in vitro fertilizing capacity of cryopreserved mouse sperm by factors released by damaged sperm, and stimulation by glutathione
Abstract
Background: In vitro fertilization (IVF) of eggs by frozen and thawed C57BL/6J mouse sperm is inhibited by dead sperm and enhanced by preincubation of the sperm in calcium-free medium. In other species, the presence of sperm killed by freezing and thawing has been associated with the generation of hydrogen peroxide.
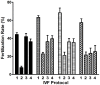
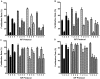
Methodology/principal findings: The proportion of eggs fertilized by cryopreserved C57BL/6J mouse sperm was increased significantly by increasing the volume of fertilization medium in which sperm and eggs were coincubated. Enhanced fertilization occurred even though the concentration of potentially fertile sperm was decreased fivefold. This suggested that if a putative soluble factor was inhibiting fertilization, dilution of that factor, but not the sperm, should increase the fertilization rate. This was achieved by coincubation of the gametes in cell culture inserts (Transwells) that during incubation were transferred progressively to wells containing fresh fertilization medium. Fertilization rates using inserts were high (66.6+/-2.4% versus 27.3%+/-2.8% in wells alone). On the assumption that the soluble factor could be H(2)O(2), reduced glutathione was added to the fertilization medium. This enhanced fertilization rate significantly (76.6%+/-2.0% versus 21.2%+/-1.9%), while addition of oxidized glutathione did not (82.7%+/-6.5% with reduced glutathione; 44.5+/-8.8% with oxidized glutathione; 47.8%+/-12.1% with no glutathione). Positive effects of reduced glutathione on IVF were also seen with frozen 129S1, FVB, and C3H sperm, and sperm from two lines of genetically modified C57BL/6J mice.
Conclusions/significance: IVF in cell culture inserts and addition of glutathione to fertilization medium significantly increased the proportion of eggs fertilized by cryopreserved mouse sperm from four inbred strains, suggesting that reactive oxygen species generated during fertilization inhibit fertilization. The modified IVF techniques developed here enhance the feasibility and efficiency of using cryopreserved sperm from genetically modified lines of inbred mice.
Conflict of interest statement
Figures

Similar articles
-
Reduced glutathione enhances fertility of frozen/thawed C57BL/6 mouse sperm after exposure to methyl-beta-cyclodextrin.Biol Reprod. 2011 Nov;85(5):1066-72. doi: 10.1095/biolreprod.111.092536. Epub 2011 Jul 20. Biol Reprod. 2011. PMID: 21778138
-
Sperm freezing and in vitro fertilization in three substrains of C57BL/6 mice.J Am Assoc Lab Anim Sci. 2009 Jan;48(1):39-43. J Am Assoc Lab Anim Sci. 2009. PMID: 19245749 Free PMC article.
-
The influence of reduced glutathione in fertilization medium on the fertility of in vitro-matured C57BL/6 mouse oocytes.Theriogenology. 2013 Sep 15;80(5):421-6. doi: 10.1016/j.theriogenology.2013.07.002. Epub 2013 Aug 2. Theriogenology. 2013. PMID: 23916252 Review.
-
Comparison of fertility between intracytoplasmic sperm injection and in vitro fertilization with a partial zona pellucida incision by using a piezo-micromanipulator in cryopreserved inbred mouse spermatozoa.Contemp Top Lab Anim Sci. 2004 Jan;43(1):21-5. Contemp Top Lab Anim Sci. 2004. PMID: 14984285
-
[Cryopreservation of embryos and gametes in mice].Jikken Dobutsu. 1994 Jan;43(1):11-8. doi: 10.1538/expanim1978.43.1_11. Jikken Dobutsu. 1994. PMID: 8119331 Review. Japanese.
Cited by
-
Wild-derived inbred mice no longer ART-resistant.Biol Reprod. 2012 May 31;86(5):168, 1-2. doi: 10.1095/biolreprod.112.100230. Print 2012 May. Biol Reprod. 2012. PMID: 22423046 Free PMC article.
-
Cryopreservation of Gametes and Embryos and Their Molecular Changes.Int J Mol Sci. 2021 Oct 8;22(19):10864. doi: 10.3390/ijms221910864. Int J Mol Sci. 2021. PMID: 34639209 Free PMC article. Review.
-
A simple and economic protocol for efficient in vitro fertilization using cryopreserved mouse sperm.PLoS One. 2021 Oct 28;16(10):e0259202. doi: 10.1371/journal.pone.0259202. eCollection 2021. PLoS One. 2021. PMID: 34710162 Free PMC article.
-
Inter-subspecies mouse F1 hybrid embryonic stem cell lines newly established for studies of allelic imbalance in gene expression.Exp Anim. 2024 Jul 9;73(3):310-318. doi: 10.1538/expanim.24-0002. Epub 2024 Mar 6. Exp Anim. 2024. PMID: 38447983 Free PMC article.
-
Age-associated aberrations of the cumulus-oocyte interaction and in the zona pellucida structure reduce fertility in female mice.Commun Biol. 2024 Dec 24;7(1):1692. doi: 10.1038/s42003-024-07305-z. Commun Biol. 2024. PMID: 39719529 Free PMC article.
References
-
- Bath ML. Simple and efficient in vitro fertilization with cryopreserved C57BL/6J mouse sperm. Biol Reprod. 2003;68:19–23. - PubMed
-
- Nishizono H, Shioda M, Takeo T, Irie T, Nakagata N. Decrease of fertilizing ability of mouse spermatozoa after freezing and thawing is related to cellular injury. Biol Reprod. 2004;71:973–978. - PubMed
-
- Suzuki-Migishima R, Hino T, Takabe M, Oda K, Migishima F, et al. Marked improvement of fertility of cryopreserved C57BL/6J mouse sperm by depletion of Ca2+ in medium. J Reprod Dev. 2009;55:386–392. - PubMed
-
- Takeo T, Hoshii T, Kondo Y, Toyodome H, Arima H, et al. Methyl-beta-cyclodextrin improves fertilizing ability of C57BL/6J mouse sperm after freezing and thawing by facilitating cholesterol efflux from the cells. Biol Reprod. 2008;78:546–551. - PubMed
-
- Taguma K, Nakamura C, Ozaki A, Suzuki C, Hachisu A, et al. A practical novel method for ensuring stable capacitation of spermatozoa from cryopreserved C57BL/6J sperm suspension. Exp Anim. 2009;58:395–401. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

