The Drosophila gene CheB42a is a novel modifier of Deg/ENaC channel function
- PMID: 20195381
- PMCID: PMC2827562
- DOI: 10.1371/journal.pone.0009395
The Drosophila gene CheB42a is a novel modifier of Deg/ENaC channel function
Abstract
Degenerin/epithelial Na(+) channels (DEG/ENaC) represent a diverse family of voltage-insensitive cation channels whose functions include Na(+) transport across epithelia, mechanosensation, nociception, salt sensing, modification of neurotransmission, and detecting the neurotransmitter FMRFamide. We previously showed that the Drosophila melanogaster Deg/ENaC gene lounge lizard (llz) is co-transcribed in an operon-like locus with another gene of unknown function, CheB42a. Because operons often encode proteins in the same biochemical or physiological pathway, we hypothesized that CHEB42A and LLZ might function together. Consistent with this hypothesis, we found both genes expressed in cells previously implicated in sensory functions during male courtship. Furthermore, when coexpressed, LLZ coprecipitated with CHEB42A, suggesting that the two proteins form a complex. Although LLZ expressed either alone or with CHEB42A did not generate ion channel currents, CHEB42A increased current amplitude of another DEG/ENaC protein whose ligand (protons) is known, acid-sensing ion channel 1a (ASIC1a). We also found that CHEB42A was cleaved to generate a secreted protein, suggesting that CHEB42A may play an important role in the extracellular space. These data suggest that CHEB42A is a modulatory subunit for sensory-related Deg/ENaC signaling. These results are consistent with operon-like transcription of CheB42a and llz and explain the similar contributions of these genes to courtship behavior.
Conflict of interest statement
Figures

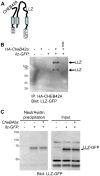

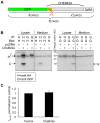
 Predicted signal peptide cleavage site. T, N, and C represent the predicted full length and cleaved products. B. CHEB42A is a secreted protein. Cells were transfected with tagged cDNA (CheB42a) or pcDNA3.1 as a control. Proteins from cell lysates or cell media were immunoprecipitated (IP) with either anti-GFP (G) or anti-HA (H) antibodies, and then blotted for either tag. T, N, and C indicate the predicted peptides from Fig. 7A. C. Effects of CheB42a-conditioned media on ASIC1a currents. ASIC1a was expressed in Xenopus oocytes as in Fig. 6. Four ASIC1a-expressing oocytes were tested for pH-dependent currents with and without conditioned media from CheB42a-expressing cells. The same oocytes (N = 4) were stimulated with unconditioned medium (pH 5.5), followed by stimulation with CheB42a-conditioned medium. There was no obvious effect of the conditioned media on pH-activation of ASIC1a channels. Currents were normalized in the conditioned state relative to the control media. All oocytes showed significant ASIC1a-dependent currents in response to pH 5.5, which does not elicit any currents in non-injected oocytes.
Predicted signal peptide cleavage site. T, N, and C represent the predicted full length and cleaved products. B. CHEB42A is a secreted protein. Cells were transfected with tagged cDNA (CheB42a) or pcDNA3.1 as a control. Proteins from cell lysates or cell media were immunoprecipitated (IP) with either anti-GFP (G) or anti-HA (H) antibodies, and then blotted for either tag. T, N, and C indicate the predicted peptides from Fig. 7A. C. Effects of CheB42a-conditioned media on ASIC1a currents. ASIC1a was expressed in Xenopus oocytes as in Fig. 6. Four ASIC1a-expressing oocytes were tested for pH-dependent currents with and without conditioned media from CheB42a-expressing cells. The same oocytes (N = 4) were stimulated with unconditioned medium (pH 5.5), followed by stimulation with CheB42a-conditioned medium. There was no obvious effect of the conditioned media on pH-activation of ASIC1a channels. Currents were normalized in the conditioned state relative to the control media. All oocytes showed significant ASIC1a-dependent currents in response to pH 5.5, which does not elicit any currents in non-injected oocytes.References
-
- Lawrence JG. Gene organization: selection, selfishness, and serendipity. Annu Rev Microbiol. 2003;57:419–440. - PubMed
-
- Blumenthal T, Gleason KS. Caenorhabditis elegans operons: form and function. Nat Rev Genet. 2003;4:112–120. - PubMed
-
- Lawrence JG. Shared strategies in gene organization among prokaryotes and eukaryotes. Cell. 2002;110:407–413. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

