Immune physiology in tissue regeneration and aging, tumor growth, and regenerative medicine
- PMID: 20195382
- PMCID: PMC2830052
- DOI: 10.18632/aging.100024
Immune physiology in tissue regeneration and aging, tumor growth, and regenerative medicine
Abstract
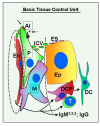
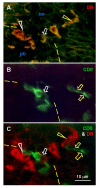
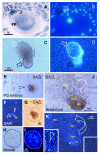
The immune system plays an important role in immunity (immune surveillance), but also in the regulation of tissue homeostasis (immune physiology). Lessons from the female reproductive tract indicate that immune system related cells, such as intraepithelial T cells and monocyte-derived cells (MDC) in stratified epithelium, interact amongst themselves and degenerate whereas epithelial cells proliferate and differentiate. In adult ovaries, MDC and T cells are present during oocyte renewal from ovarian stem cells. Activated MDC are also associated with follicular development and atresia, and corpus luteum differentiation. Corpus luteum demise resembles rejection of a graft since it is attended by a massive influx of MDC and T cells resulting in parenchymal and vascular regression. Vascular pericytes play important roles in immune physiology, and their activities (including secretion of the Thy-1 differentiation protein) can be regulated by vascular autonomic innervation. In tumors, MDC regulate proliferation of neoplastic cells and angiogenesis. Tumor infiltrating T cells die among malignant cells. Alterations of immune physiology can result in pathology, such as autoimmune, metabolic, and degenerative diseases, but also in infertility and intrauterine growth retardation, fetal morbidity and mortality. Animal experiments indicate that modification of tissue differentiation (retardation or acceleration) during immune adaptation can cause malfunction (persistent immaturity or premature aging) of such tissue during adulthood. Thus successful stem cell therapy will depend on immune physiology in targeted tissues. From this point of view, regenerative medicine is more likely to be successful in acute rather than chronic tissue disorders.
Keywords: aging; animal models; follicular renewal; immune physiology; mesenchymal-epithelial interactions; proliferation; regeneration; regenerative medicine; tissue homeostasis; tumor growth.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures









Similar articles
-
Immunoregulation of follicular renewal, selection, POF, and menopause in vivo, vs. neo-oogenesis in vitro, POF and ovarian infertility treatment, and a clinical trial.Reprod Biol Endocrinol. 2012 Nov 23;10:97. doi: 10.1186/1477-7827-10-97. Reprod Biol Endocrinol. 2012. PMID: 23176151 Free PMC article. Review.
-
Immune maintenance of self in morphostasis of distinct tissues, tumour growth and regenerative medicine.Scand J Immunol. 2011 Mar;73(3):159-89. doi: 10.1111/j.1365-3083.2010.02497.x. Scand J Immunol. 2011. PMID: 21204896 Review.
-
Immune system involvement in the regulation of ovarian function and augmentation of cancer.Microsc Res Tech. 2006 Jun;69(6):482-500. doi: 10.1002/jemt.20307. Microsc Res Tech. 2006. PMID: 16703613 Review.
-
Association of mesenchymal cells and immunoglobulins with differentiating epithelial cells.BMC Dev Biol. 2001;1:11. doi: 10.1186/1471-213x-1-11. Epub 2001 Jun 22. BMC Dev Biol. 2001. PMID: 11439174 Free PMC article.
-
Immune physiology of the mammalian ovary - a review.Am J Reprod Immunol. 2008 Jan;59(1):12-26. doi: 10.1111/j.1600-0897.2007.00562.x. Am J Reprod Immunol. 2008. PMID: 18154592 Review.
Cited by
-
Functional differences between neonatal and adult fibroblasts and keratinocytes: Donor age affects epithelial-mesenchymal crosstalk in vitro.Int J Mol Med. 2016 Oct;38(4):1063-74. doi: 10.3892/ijmm.2016.2706. Epub 2016 Aug 11. Int J Mol Med. 2016. PMID: 27513730 Free PMC article.
-
Skin healing and scale regeneration in fed and unfed sea bream, Sparus auratus.BMC Genomics. 2011 Oct 7;12:490. doi: 10.1186/1471-2164-12-490. BMC Genomics. 2011. PMID: 21981800 Free PMC article.
-
CD90 and CD24 Co-Expression Is Associated with Pancreatic Intraepithelial Neoplasias.PLoS One. 2016 Jun 22;11(6):e0158021. doi: 10.1371/journal.pone.0158021. eCollection 2016. PLoS One. 2016. PMID: 27332878 Free PMC article.
-
Immunoregulation of follicular renewal, selection, POF, and menopause in vivo, vs. neo-oogenesis in vitro, POF and ovarian infertility treatment, and a clinical trial.Reprod Biol Endocrinol. 2012 Nov 23;10:97. doi: 10.1186/1477-7827-10-97. Reprod Biol Endocrinol. 2012. PMID: 23176151 Free PMC article. Review.
-
Thy-1 Regulates VEGF-Mediated Choroidal Endothelial Cell Activation and Migration: Implications in Neovascular Age-Related Macular Degeneration.Invest Ophthalmol Vis Sci. 2016 Oct 1;57(13):5525-5534. doi: 10.1167/iovs.16-19691. Invest Ophthalmol Vis Sci. 2016. PMID: 27768790 Free PMC article.
References
-
- Fidler IJ. Lymphocytes are not only immunocytes. Biomedicine. 1980;32:1–3. - PubMed
-
- Bukovsky A, Caudle MR, Keenan JA. Dominant role of monocytes in control of tissue function and aging. Med Hypotheses. 2000;55:337–347. - PubMed
-
- Havran WL, Jameson JM, Witherden DA. Epithelial cells and their neighbors. III. Interactions between intraepithelial lymphocytes and neighboring epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:G627–G630. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
