Modeling of replicative senescence in hematopoietic development
- PMID: 20195386
- PMCID: PMC2830082
- DOI: 10.18632/aging.100072
Modeling of replicative senescence in hematopoietic development
Abstract
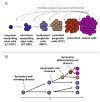
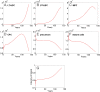
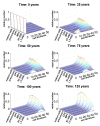
Hematopoietic stem cells (HSC) give rise to an enormous number of blood cells throughout our life. In contrast their number of cell divisions preceding senescence is limited underin vitro culture conditions. Here we consider the question whether HSC can rejuvenate indefinitely or if the number of cell divisions is restricted. We have developed a multi-compartmental model for hematopoietic differentiation based on ordinary differential equations. The model is based on the hypothesis that in each step of maturation, the percentage of self-renewal versus differentiation is regulated by a single external feedback mechanism. We simulate the model under the assumption that hematopoietic differentiation precedes the six steps of maturation and the cells ultimately cease to proliferate after 50 divisions. Our results demonstrate that it is conceivable to maintain hematopoiesis over a life-time if HSC have a slow division rate and a high self-renewal rate. With age, the feedback signal increases and this enhances self-renewal, which results in the increase of the number of stem and progenitor cells. This study demonstrates that replicative senescence is compatible with life-long hematopoiesis and that model predictions are in line with experimental observations. Thus, HSC might not divide indefinitely with potentially important clinical implications.
Keywords: aging; hematopoietic stem cells; mathematical model; replicative senescence; self-renewal.
Conflict of interest statement
The authors in this manuscript have no conflict of interests to declare.
Figures

References
-
- Wagner W, Horn P, Bork S, Ho AD. Aging of hematopoietic stem cells is regulated by the stem cell niche. Experimental Gerontology. 2008;43:974–980. - PubMed
-
- Globerson A. Hematopoietic stem cells and aging. Exp Gerontol. 1999;34:137–146. - PubMed
-
- Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. - PubMed
-
- Kim M, Moon HB, Spangrude GJ. Major age-related changes of mouse hematopoietic stem/progenitor cells. Ann N Y Acad Sci. 2003;996:195–208. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
