CCN3/CCN2 regulation and the fibrosis of diabetic renal disease
- PMID: 20195391
- PMCID: PMC2821477
- DOI: 10.1007/s12079-010-0085-z
CCN3/CCN2 regulation and the fibrosis of diabetic renal disease
Abstract
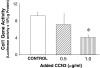
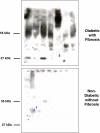
Prior work in the CCN field, including our own, suggested to us that there might be co-regulatory activity and function as part of the actions of this family of cysteine rich cytokines. CCN2 is now regarded as a major pro-fibrotic molecule acting both down-stream and independent of TGF-beta1, and appears causal in the disease afflicting multiple organs. Since diabetic renal fibrosis is a common complication of diabetes, and a major cause of end stage renal disease (ESRD), we examined the possibility that CCN3 (NOV), might act as an endogenous negative regulator of CCN2 with the capacity to limit the overproduction of extracellular matrix (ECM), and thus prevent, or ameliorate fibrosis. We demonstrate, using an in vitro model of diabetic renal fibrosis, that both exogenous treatment with CCN3 and transfection with the over-expression of the CCN3 gene in mesangial cells markedly down-regulates CCN2 activity and blocks ECM over-accumulation stimulated by TGF-beta1. Conversely, TGF-beta1 treatment reduces endogenous CCN3 expression and increases CCN2 activity and matrix accumulation, indicating an important, novel yin/yang effect. Using the db/db mouse model of diabetic nephropathy, we confirm the expression of CCN3 in the kidney, with temporal localization that supports these in vitro findings. In summary, the results corroborate our hypothesis that one function of CCN3 is to regulate CCN2 activity and at the concentrations and conditions used down-regulates the effects of TGF-beta1, acting to limit ECM turnover and fibrosis in vivo. The findings suggest opportunities for novel endogenous-based therapy either by the administration, or the upregulation of CCN3.
Keywords: Anti-fibrotic therapy; CCN regulation; Diabetic nephropathy; Fibrosis.
Figures







References
-
- Brigstock DR, Steffen CL, Kim GY, Vegunta RK, Diehl JR, Harding PA. Purification and characterization of novel heparin-binding growth factors in uterine secretory fluids. Identification as heparin-regulated Mr 10,000 forms of connective tissue growth factor. J Biol Chem. 1997;272(32):20275–20282. doi: 10.1074/jbc.272.32.20275. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

