Quantitative comparison of HTLV-1 and HIV-1 cell-to-cell infection with new replication dependent vectors
- PMID: 20195464
- PMCID: PMC2829072
- DOI: 10.1371/journal.ppat.1000788
Quantitative comparison of HTLV-1 and HIV-1 cell-to-cell infection with new replication dependent vectors
Abstract
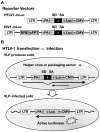
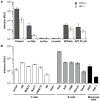
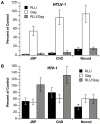
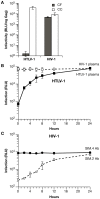
We have developed an efficient method to quantify cell-to-cell infection with single-cycle, replication dependent reporter vectors. This system was used to examine the mechanisms of infection with HTLV-1 and HIV-1 vectors in lymphocyte cell lines. Effector cells transfected with reporter vector, packaging vector, and Env expression plasmid produced virus-like particles that transduced reporter gene activity into cocultured target cells with zero background. Reporter gene expression was detected exclusively in target cells and required an Env-expression plasmid and a viral packaging vector, which provided essential structural and enzymatic proteins for virus replication. Cell-cell fusion did not contribute to infection, as reporter protein was rarely detected in syncytia. Coculture of transfected Jurkat T cells and target Raji/CD4 B cells enhanced HIV-1 infection two fold and HTLV-1 infection ten thousand fold in comparison with cell-free infection of Raji/CD4 cells. Agents that interfere with actin and tubulin polymerization strongly inhibited HTLV-1 and modestly decreased HIV-1 cell-to-cell infection, an indication that cytoskeletal remodeling was more important for HTLV-1 transmission. Time course studies showed that HTLV-1 transmission occurred very rapidly after cell mixing, whereas slower kinetics of HIV-1 coculture infection implies a different mechanism of infectious transmission. HTLV-1 Tax was demonstrated to play an important role in altering cell-cell interactions that enhance virus infection and replication. Interestingly, superantigen-induced synapses between Jurkat cells and Raji/CD4 cells did not enhance infection for either HTLV-1 or HIV-1. In general, the dependence on cell-to-cell infection was determined by the virus, the effector and target cell types, and by the nature of the cell-cell interaction.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Phillips DM. The role of cell-to-cell transmission in HIV infection. Aids. 1994;8:719–731. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

