Cyr61/CCN1 displays high-affinity binding to the somatomedin B(1-44) domain of vitronectin
- PMID: 20195466
- PMCID: PMC2829074
- DOI: 10.1371/journal.pone.0009356
Cyr61/CCN1 displays high-affinity binding to the somatomedin B(1-44) domain of vitronectin
Abstract
Background: Cyr61 is a member of the CCN (Cyr61, connective tissue growth, NOV) family of extracellular-associated (matricellular) proteins that present four distinct functional modules, namely insulin-like growth factor binding protein (IGFBP), von Willebrand factor type C (vWF), thrombospondin type 1 (TSP), and C-terminal growth factor cysteine knot (CT) domain. While heparin sulphate proteoglycans reportedly mediate the interaction of Cyr61 with the matrix and cell surface, the role of other extracellular associated proteins has not been revealed.
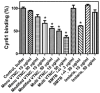
Methods and findings: In this report, surface plasmon resonance (SPR) experiments and solid-phase binding assays demonstrate that recombinant Cyr61 interacts with immobilized monomeric or multimeric vitronectin (VTNC) with K(D) in the nanomolar range. Notably, the binding site for Cyr61 was identified as the somatomedin B domain (SMTB(1-44)) of VTNC, which mediates its interaction with PAI-1, uPAR, and integrin alphav beta3. Accordingly, PAI-1 outcompetes Cyr61 for binding to immobilized SMTB(1-44), and Cyr61 attenuates uPAR-mediated U937 adhesion to VTNC. In contrast, isothermal titration calorimetry shows that Cyr61 does not display high-affinity binding for SMTB(1-44) in solution. Nevertheless, competitive ELISA revealed that multimeric VTNC, heat-modified monomeric VTNC, or SMTB(1-44) at high concentrations attenuate Cyr61 binding to immobilized VTNC, while monomeric VTNC was ineffective. Therefore, immobilization of VTNC exposes cryptic epitopes that recognize Cyr61 with high affinity, as reported for a number of antibodies, beta-endorphin, and other molecules.
Conclusions: The finding that Cyr61 interacts with the SMTB(1-44) domain suggests that VTNC represent a point of anchorage for CCN family members to the matrix. Results are discussed in the context of the role of CCN and VTNC in matrix biology and angiogenesis.
Conflict of interest statement
Figures





References
-
- Brigstock DR. The connective tissue growth factor/cysteine-rich 61/nephroblastoma overexpressed (CCN) family. Endocr Rev. 1999;20:189–206. - PubMed
-
- Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119:4803–4810. - PubMed
-
- Bach LA, Headey SJ, Norton RS. IGF-binding proteins—the pieces are falling into place. Trends Endocrinol Metab. 2005;16:228–234. - PubMed
-
- Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23:824–854. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

