Differentiation of glioma and radiation injury in rats using in vitro produce magnetically labeled cytotoxic T-cells and MRI
- PMID: 20195476
- PMCID: PMC2829084
- DOI: 10.1371/journal.pone.0009365
Differentiation of glioma and radiation injury in rats using in vitro produce magnetically labeled cytotoxic T-cells and MRI
Abstract
Background: A limitation with current imaging strategies of recurrent glioma undergoing radiotherapy is that tumor and radiation injury cannot be differentiated with post contrast CT or MRI, or with PET or other more complex parametric analyses of MRI data. We propose to address the imaging limitation building on emerging evidence indicating that effective therapy for recurrent glioma can be attained by sensitized T-cells following vaccination of primed dendritic cells (DCs). The purpose of this study was to determine whether cord blood T-cells can be sensitized against glioma cells (U-251) and if these sensitized cytotoxic T-cells (CTLs) can be used as cellular magnetic resonance imaging probes to identify and differentiate glioma from radiation necrosis in rodent models.
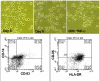
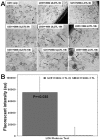
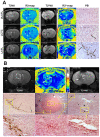
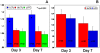
Methodology/principal findings: Cord blood T and CD14+ cells were collected. Isolated CD14+ cells were then converted to dendritic cells (DCs), primed with glioma cell lysate and used to sensitize T-cells. Phenotypical expression of the generated DCs were analyzed to determine the expression level of CD14, CD86, CD83 and HLA-DR. Cells positive for CD25, CD4, CD8 were determined in generated CTLs. Specificity of cytotoxicity of the generated CTLs was also determined by lactate dehydrogenase (LDH) release assay. Secondary proliferation capacity of magnetically labeled and unlabeled CTLs was also determined. Generated CTLs were magnetically labeled and intravenously injected into glioma bearing animals that underwent MRI on days 3 and 7 post- injection. CTLs were also administered to animals with focal radiation injury to determine whether these CTLs accumulated non-specifically to the injury sites. Multi-echo T2- and T2*-weighted images were acquired and R2 and R2* maps created. Our method produced functional, sensitized CTLs that specifically induced U251 cell death in vitro. Both labeled and unlabeled CTLs proliferated equally after the secondary stimulation. There were significantly higher CD25 positive cells (p = <0.006) in CTLs. In addition, T2- and T2*-weighted MR images showed increased low signal intensity areas in animals that received labeled CTLs as compared to the images from animals that received control cells. Histological analysis confirmed the presence of iron positive cells in sites corresponding to MRI low signal intensity regions. Significant differences (p = <0.001) in tumor R2 and R2* values were observed among the groups of animals. Animals with radiation injury exhibited neither MRI hypointense areas nor presence of iron positive cells.
Conclusion: Our results indicate that T-cells can be effectively sensitized by in vitro methods and used as cellular probes to identify and differentiate glioma from radiation necrosis.
Conflict of interest statement
Figures



References
-
- Remer S, Murphy ME. The challenges of long-term treatment outcomes in adults with malignant gliomas. Clin J Oncol Nurs. 2004;8:368–376. - PubMed
-
- Iwama T, Yamada H, Sakai N, Andoh T, Nakashima T, et al. Correlation between magnetic resonance imaging and histopathology of intracranial glioma. Neurol Res. 1991;13:48–54. - PubMed
-
- Watanabe M, Tanaka R, Takeda N. Magnetic resonance imaging and histopathology of cerebral gliomas. Neuroradiology. 1992;34:463–469. - PubMed
-
- Dhermain F, Ducreux D, Bidault F, Bruna A, Parker F, et al. [Use of the functional imaging modalities in radiation therapy treatment planning in patients with glioblastoma]. Bull Cancer. 2005;92:333–342. - PubMed
-
- Chang SM, Butowski NA, Sneed PK, Garner IV. Standard treatment and experimental targeted drug therapy for recurrent glioblastoma multiforme. Neurosurg Focus. 2006;20:E4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

