Local membrane deformations activate Ca2+-dependent K+ and anionic currents in intact human red blood cells
- PMID: 20195477
- PMCID: PMC2829085
- DOI: 10.1371/journal.pone.0009447
Local membrane deformations activate Ca2+-dependent K+ and anionic currents in intact human red blood cells
Abstract
Background: The mechanical, rheological and shape properties of red blood cells are determined by their cortical cytoskeleton, evolutionarily optimized to provide the dynamic deformability required for flow through capillaries much narrower than the cell's diameter. The shear stress induced by such flow, as well as the local membrane deformations generated in certain pathological conditions, such as sickle cell anemia, have been shown to increase membrane permeability, based largely on experimentation with red cell suspensions. We attempted here the first measurements of membrane currents activated by a local and controlled membrane deformation in single red blood cells under on-cell patch clamp to define the nature of the stretch-activated currents.
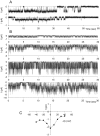
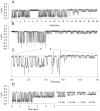
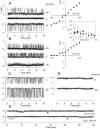
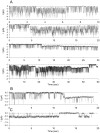
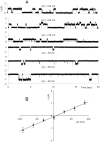
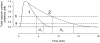
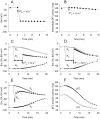
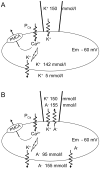
Methodology/principal findings: The cell-attached configuration of the patch-clamp technique was used to allow recordings of single channel activity in intact red blood cells. Gigaohm seal formation was obtained with and without membrane deformation. Deformation was induced by the application of a negative pressure pulse of 10 mmHg for less than 5 s. Currents were only detected when the membrane was seen domed under negative pressure within the patch-pipette. K(+) and Cl(-) currents were strictly dependent on the presence of Ca(2+). The Ca(2+)-dependent currents were transient, with typical decay half-times of about 5-10 min, suggesting the spontaneous inactivation of a stretch-activated Ca(2+) permeability (PCa). These results indicate that local membrane deformations can transiently activate a Ca(2+) permeability pathway leading to increased [Ca(2+)](i), secondary activation of Ca(2+)-sensitive K(+) channels (Gardos channel, IK1, KCa3.1), and hyperpolarization-induced anion currents.
Conclusions/significance: The stretch-activated transient PCa observed here under local membrane deformation is a likely contributor to the Ca(2+)-mediated effects observed during the normal aging process of red blood cells, and to the increased Ca(2+) content of red cells in certain hereditary anemias such as thalassemia and sickle cell anemia.
Conflict of interest statement
Figures


References
-
- Bouyer G, Egee S, Thomas SL. Three types of spontaneously active anionic channels in malaria-infected human red blood cells. Blood Cells Mol Dis. 2006;36:248–254. - PubMed
-
- Decherf G, Egee S, Staines HM, Ellory JC, Thomas SL. Anionic channels in malaria-infected human red blood cells. Blood Cells Mol Dis. 2004;32:366–371. - PubMed
-
- Decherf G, Bouyer G, Egee S, Thomas SL. Chloride channels in normal and cystic fibrosis human erythrocyte membrane. Blood Cells Mol Dis. 2007;39:24–34. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

