Cdk1 targets Srs2 to complete synthesis-dependent strand annealing and to promote recombinational repair
- PMID: 20195513
- PMCID: PMC2829061
- DOI: 10.1371/journal.pgen.1000858
Cdk1 targets Srs2 to complete synthesis-dependent strand annealing and to promote recombinational repair
Abstract
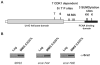
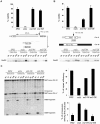
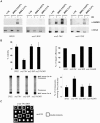
Cdk1 kinase phosphorylates budding yeast Srs2, a member of UvrD protein family, displays both DNA translocation and DNA unwinding activities in vitro. Srs2 prevents homologous recombination by dismantling Rad51 filaments and is also required for double-strand break (DSB) repair. Here we examine the biological significance of Cdk1-dependent phosphorylation of Srs2, using mutants that constitutively express the phosphorylated or unphosphorylated protein isoforms. We found that Cdk1 targets Srs2 to repair DSB and, in particular, to complete synthesis-dependent strand annealing, likely controlling the disassembly of a D-loop intermediate. Cdk1-dependent phosphorylation controls turnover of Srs2 at the invading strand; and, in absence of this modification, the turnover of Rad51 is not affected. Further analysis of the recombination phenotypes of the srs2 phospho-mutants showed that Srs2 phosphorylation is not required for the removal of toxic Rad51 nucleofilaments, although it is essential for cell survival, when DNA breaks are channeled into homologous recombinational repair. Cdk1-targeted Srs2 displays a PCNA-independent role and appears to have an attenuated ability to inhibit recombination. Finally, the recombination defects of unphosphorylatable Srs2 are primarily due to unscheduled accumulation of the Srs2 protein in a sumoylated form. Thus, the Srs2 anti-recombination function in removing toxic Rad51 filaments is genetically separable from its role in promoting recombinational repair, which depends exclusively on Cdk1-dependent phosphorylation. We suggest that Cdk1 kinase counteracts unscheduled sumoylation of Srs2 and targets Srs2 to dismantle specific DNA structures, such as the D-loops, in a helicase-dependent manner during homologous recombinational repair.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

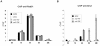
Similar articles
-
Multifunctional roles of Saccharomyces cerevisiae Srs2 protein in replication, recombination and repair.FEMS Yeast Res. 2017 Mar 1;17(2):fow111. doi: 10.1093/femsyr/fow111. FEMS Yeast Res. 2017. PMID: 28011904 Free PMC article. Review.
-
Context-dependent remodeling of Rad51-DNA complexes by Srs2 is mediated by a specific protein-protein interaction.J Mol Biol. 2014 May 1;426(9):1883-97. doi: 10.1016/j.jmb.2014.02.014. Epub 2014 Feb 24. J Mol Biol. 2014. PMID: 24576606
-
Nej1 recruits the Srs2 helicase to DNA double-strand breaks and supports repair by a single-strand annealing-like mechanism.Proc Natl Acad Sci U S A. 2009 Jul 21;106(29):12037-42. doi: 10.1073/pnas.0903869106. Epub 2009 Jul 1. Proc Natl Acad Sci U S A. 2009. PMID: 19571008 Free PMC article.
-
The Srs2 helicase activity is stimulated by Rad51 filaments on dsDNA: implications for crossover incidence during mitotic recombination.Mol Cell. 2008 Feb 1;29(2):243-54. doi: 10.1016/j.molcel.2007.11.033. Mol Cell. 2008. PMID: 18243118
-
PCNASUMO and Srs2: a model SUMO substrate-effector pair.Biochem Soc Trans. 2007 Dec;35(Pt 6):1385-8. doi: 10.1042/BST0351385. Biochem Soc Trans. 2007. PMID: 18031227 Review.
Cited by
-
Pro-recombination Role of Srs2 Protein Requires SUMO (Small Ubiquitin-like Modifier) but Is Independent of PCNA (Proliferating Cell Nuclear Antigen) Interaction.J Biol Chem. 2016 Apr 1;291(14):7594-607. doi: 10.1074/jbc.M115.685891. Epub 2016 Feb 9. J Biol Chem. 2016. PMID: 26861880 Free PMC article.
-
RNA-processing proteins regulate Mec1/ATR activation by promoting generation of RPA-coated ssDNA.EMBO Rep. 2015 Feb;16(2):221-31. doi: 10.15252/embr.201439458. Epub 2014 Dec 19. EMBO Rep. 2015. PMID: 25527408 Free PMC article.
-
SUMOylation of Rad52-Rad59 synergistically change the outcome of mitotic recombination.DNA Repair (Amst). 2016 Jun;42:11-25. doi: 10.1016/j.dnarep.2016.04.001. Epub 2016 Apr 16. DNA Repair (Amst). 2016. PMID: 27130983 Free PMC article.
-
Post-replication repair suppresses duplication-mediated genome instability.PLoS Genet. 2010 May 6;6(5):e1000933. doi: 10.1371/journal.pgen.1000933. PLoS Genet. 2010. PMID: 20463880 Free PMC article.
-
An overview of Cdk1-controlled targets and processes.Cell Div. 2010 May 13;5:11. doi: 10.1186/1747-1028-5-11. Cell Div. 2010. PMID: 20465793 Free PMC article.
References
-
- Keen-Kim D, Nooraie F, Rao PN. Cytogenetic biomarkers for human cancer. Front Biosci. 2008;13:5928–5949. - PubMed
-
- Krogh BO, Symington LS. Recombination proteins in yeast. Annu Rev Genet. 2004;38:233–271. - PubMed
-
- San Filippo J, Sung P, Klein H. Mechanism of eukaryotic homologous recombination. Annu Rev Biochem. 2008;77:229–257. - PubMed
-
- Liu Y, West SC. Happy Hollidays: 40th anniversary of the Holliday junction. Nat Rev Mol Cell Biol. 2004;5:937–944. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

