Broadly protective monoclonal antibodies against H3 influenza viruses following sequential immunization with different hemagglutinins
- PMID: 20195520
- PMCID: PMC2829068
- DOI: 10.1371/journal.ppat.1000796
Broadly protective monoclonal antibodies against H3 influenza viruses following sequential immunization with different hemagglutinins
Abstract
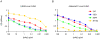
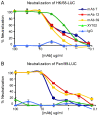
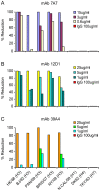
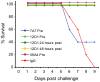
As targets of adaptive immunity, influenza viruses are characterized by the fluidity with which they respond to the selective pressure applied by neutralizing antibodies. This mutability of structural determinants of protective immunity is the obstacle in developing universal influenza vaccines. Towards the development of such vaccines and other immune therapies, our studies are designed to identify regions of influenza viruses that are conserved and that mediate virus neutralization. We have specifically focused on viruses of the H3N2 subtype, which have persisted as a principal source of influenza-related morbidity and mortality in humans since the pandemic of 1968. Three monoclonal antibodies have been identified that are broadly-neutralizing against H3 influenza viruses spanning 40 years. The antibodies react with the hemagglutinin glycoprotein and appear to bind in regions that are refractory to the structural variation required for viral escape from neutralization. The antibodies demonstrate therapeutic efficacy in mice against H3N2 virus infection and have potential for use in the treatment of human influenza disease. By mapping the binding region of one antibody, 12D1, we have identified a continuous region of the hemagglutinin that may act as an immunogen to elicit broadly protective immunity to H3 viruses. The anti-H3 monoclonal antibodies were identified after immunization of mice with the hemagglutinin of four different viruses (A/Hong Kong/1/1968, A/Alabama/1/1981, A/Beijing/47/1992, A/Wyoming/3/2003). This immunization schedule was designed to boost B cells specific for conserved regions of the hemagglutinin from distinct antigenic clusters. Importantly, our antibodies are of naturally occurring specificity rather than selected from cloned libraries, demonstrating that broad-spectrum humoral immunity to influenza viruses can be elicited in vivo.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


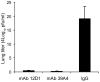
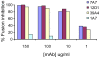

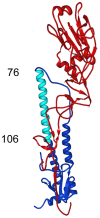
References
-
- WHO. April, 2009. Influenza (Seasonal) Fact sheet 211.
-
- Piot P, Bartos M, Ghys PD, Walker N, Schwartlander B. The global impact of HIV/AIDS. Nature. 2001;410:968–973. - PubMed
-
- Chowell G, Bertozzi SM, Colchero MA, Lopez-Gatell H, Alpuche-Aranda C, et al. Severe Respiratory Disease Concurrent with the Circulation of H1N1 Influenza. N Engl J Med 2009 - PubMed
-
- CDC Seasonal flu; United States Surveillance Data.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

