MYC overexpression induces prostatic intraepithelial neoplasia and loss of Nkx3.1 in mouse luminal epithelial cells
- PMID: 20195545
- PMCID: PMC2828486
- DOI: 10.1371/journal.pone.0009427
MYC overexpression induces prostatic intraepithelial neoplasia and loss of Nkx3.1 in mouse luminal epithelial cells
Abstract
Lo-MYC and Hi-MYC mice develop prostatic intraepithelial neoplasia (PIN) and prostatic adenocarcinoma as a result of MYC overexpression in the mouse prostate. However, prior studies have not determined precisely when, and in which cell types, MYC is induced. Using immunohistochemistry (IHC) to localize MYC expression in Lo-MYC transgenic mice, we show that morphological and molecular alterations characteristic of high grade PIN arise in luminal epithelial cells as soon as MYC overexpression is detected. These changes include increased nuclear and nucleolar size and large scale chromatin remodeling. Mouse PIN cells retained a columnar architecture and abundant cytoplasm and appeared as either a single layer of neoplastic cells or as pseudo-stratified/multilayered structures with open glandular lumina-features highly analogous to human high grade PIN. Also using IHC, we show that the onset of MYC overexpression and PIN development coincided precisely with decreased expression of the homeodomain transcription factor and tumor suppressor, Nkx3.1. Virtually all normal appearing prostate luminal cells expressed high levels of Nkx3.1, but all cells expressing MYC in PIN lesions showed marked reductions in Nkx3.1, implicating MYC as a key factor that represses Nkx3.1 in PIN lesions. To determine the effects of less pronounced overexpression of MYC we generated a new line of mice expressing MYC in the prostate under the transcriptional control of the mouse Nkx3.1 control region. These "Super-Lo-MYC" mice also developed PIN, albeit a less aggressive form. We also identified a histologically defined intermediate step in the progression of mouse PIN into invasive adenocarcinoma. These lesions are characterized by a loss of cell polarity, multi-layering, and cribriform formation, and by a "paradoxical" increase in Nkx3.1 protein. Similar histopathological changes occurred in Hi-MYC mice, albeit with accelerated kinetics. Our results using IHC provide novel insights that support the contention that MYC overexpression is sufficient to transform prostate luminal epithelial cells into PIN cells in vivo. We also identified a novel histopathologically identifiable intermediate step prior to invasion that should facilitate studies of molecular pathway alterations occurring during early progression of prostatic adenocarcinomas.
Conflict of interest statement
Figures


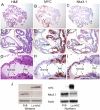

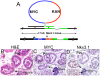
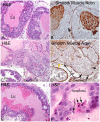


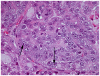
References
-
- Ellwood-Yen K, Graeber TG, Wongvipat J, Iruela-Arispe ML, Zhang J, et al. Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell. 2003;4:223–238. - PubMed
-
- Abate-Shen C, Shen MM. Molecular genetics of prostate cancer. Genes Dev. 2000;14:2410–2434. - PubMed
-
- Elo JP, Visakorpi T. Molecular genetics of prostate cancer. Ann Med. 2001;33:130–141. - PubMed
-
- Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med. 2003;349:366–381. - PubMed
-
- Gonzalgo ML, Isaacs WB. Molecular pathways to prostate cancer. J Urol. 2003;170:2444–2452. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

