An Approach for determining antibiotic loading for a physician-directed antibiotic-loaded PMMA bone cement formulation
- PMID: 20195806
- PMCID: PMC2895843
- DOI: 10.1007/s11999-010-1281-0
An Approach for determining antibiotic loading for a physician-directed antibiotic-loaded PMMA bone cement formulation
Abstract
Background: When a physician-directed antibiotic-loaded polymethylmethacrylate (PMMA) bone cement (ALBC) formulation is used in total hip arthroplasties (THAs) and total knee arthroplasties (TKAs), current practice in the United States involves arbitrary choice of the antibiotic loading (herein defined as the ratio of the mass of the antibiotic added to the mass of the cement powder). We suggest there is a need to develop a rational method for determining this loading.
Questions/purposes: We propose a new method for determining the antibiotic loading to use when preparing a physician-directed ALBC formulation and illustrate this method using three in vitro properties of an ALBC in which the antibiotic was daptomycin.
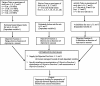
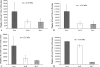
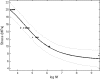
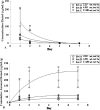
Materials and methods: Daptomycin was blended with the powder of the cement using a mechanical mixer. We performed fatigue, elution, and activity tests on three sets of specimens having daptomycin loadings of 2.25, 4.50, and 11.00 wt/wt%. Correlational analyses of the results of these tests were used in conjunction with stated constraints and a nonlinear optimization method to determine the daptomycin loading to use.
Results: With an increase in daptomycin loading, the estimated mean fatigue limit of the cement decreased, the estimated elution rate of the antibiotic increased, and the percentage inhibition of staphylococcal growth by the eluate remained unchanged at 100%. For a daptomycin-loaded PMMA bone cement we computed the optimum amount of daptomycin to mechanically blend with 40 g of cement powder is 1.36 g.
Conclusions: We suggest an approach that may be used to determine the amount of antibiotic to blend with the powder of a PMMA bone cement when preparing a physician-directed ALBC formulation, and highlighted the attractions and limitations of this approach.
Clinical relevance: When a physician-directed ALBC formulation is selected for use in a TKA or THA, the approach we detail may be employed to determine the antibiotic loading to use rather than the empirical approach that is taken in current clinical practice.
Figures

Similar articles
-
Estimation of the optimum loading of an antibiotic powder in an acrylic bone cement: gentamicin sulfate in SmartSet HV.Acta Orthop. 2006 Aug;77(4):622-7. doi: 10.1080/17453670610012700. Acta Orthop. 2006. PMID: 16929440
-
Characterization of daptomycin-loaded antibiotic cement.Orthopedics. 2012 Apr;35(4):e503-9. doi: 10.3928/01477447-20120327-19. Orthopedics. 2012. PMID: 22495850
-
Daptomycin-loaded polymethylmethacrylate bone cement for joint arthroplasty surgery.Artif Organs. 2014 Jun;38(6):484-92. doi: 10.1111/aor.12197. Epub 2013 Oct 29. Artif Organs. 2014. PMID: 24571555
-
Antibiotic-loaded bone cement and periprosthetic joint infection.J Long Term Eff Med Implants. 2014;24(2-3):89-97. doi: 10.1615/jlongtermeffmedimplants.2013010238. J Long Term Eff Med Implants. 2014. PMID: 25272207 Review.
-
The Hidden Cost of Commercial Antibiotic-Loaded Bone Cement: A Systematic Review of Clinical Results and Cost Implications Following Total Knee Arthroplasty.J Arthroplasty. 2018 Dec;33(12):3789-3792. doi: 10.1016/j.arth.2018.08.009. Epub 2018 Aug 13. J Arthroplasty. 2018. PMID: 30217400
Cited by
-
Methicillin-resistant Staphylococcal periprosthetic joint infections can be effectively controlled by systemic and local daptomycin.BMC Infect Dis. 2016 Feb 1;16:48. doi: 10.1186/s12879-016-1366-9. BMC Infect Dis. 2016. PMID: 26830838 Free PMC article.
-
Not all approved antibiotic-loaded PMMA bone cement brands are the same: ranking using the utility materials selection concept.J Mater Sci Mater Med. 2015 Jan;26(1):5388. doi: 10.1007/s10856-015-5388-4. Epub 2015 Jan 17. J Mater Sci Mater Med. 2015. PMID: 25595722
-
Antibiotic-free antimicrobial poly (methyl methacrylate) bone cements: A state-of-the-art review.World J Orthop. 2022 Apr 18;13(4):339-353. doi: 10.5312/wjo.v13.i4.339. eCollection 2022 Apr 18. World J Orthop. 2022. PMID: 35582158 Free PMC article. Review.
-
Evaluation of comparative soft tissue response to bone void fillers with antibiotics in a rabbit intramuscular model.J Biomater Appl. 2019 Jul;34(1):117-129. doi: 10.1177/0885328219838382. Epub 2019 Apr 16. J Biomater Appl. 2019. PMID: 30987506 Free PMC article.
-
Novel Antibiotic-loaded Point-of-care Implant Coating Inhibits Biofilm.Clin Orthop Relat Res. 2015 Jul;473(7):2270-82. doi: 10.1007/s11999-014-4130-8. Clin Orthop Relat Res. 2015. PMID: 25604874 Free PMC article.
References
-
- American Society for Testing and Materials (ASTM). Standard F 2118-03: Standard test method for constant amplitude of force controlled fatigue testing of acrylic bone cement materials. In: Annual Book of ASTM Standards 2008, Vol 13.01. West Conshohocken, PA: ASTM International; 2008:1136–1143.
-
- Australian Orthopaedics Association. National Joint Replacement Registry. Annual Report. Adelaide, Australia: AOA; 2009:169–177.
-
- Bates DM, Watts DG. Nonlinear Regression and Its Applications. New York, NY: Wiley; 1988.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

