[2]Catenanes decorated with porphyrin and [60]fullerene groups: design, convergent synthesis, and photoinduced processes
- PMID: 20196597
- PMCID: PMC2862559
- DOI: 10.1021/ja910149f
[2]Catenanes decorated with porphyrin and [60]fullerene groups: design, convergent synthesis, and photoinduced processes
Abstract
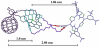
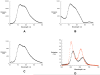
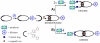
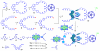
A new class of [2]catenanes containing zinc(II)-porphyrin (ZnP) and/or [60]fullerene (C(60)) as appended groups has been prepared. A complete description of the convergent synthetic approach based on Cu(I) template methodology and "click" 1,3-dipolar cycloaddition chemistry is described. This new electron donor-acceptor catenane family has been subjected to extensive spectroscopic, computational, electrochemical and photophysical studies. (1)H NMR spectroscopy and computational analysis have revealed that the ZnP-C(60)-[2]catenane adopts an extended conformation with the chromophores as far as possible from each other. A detailed photophysical investigation has revealed that upon irradiation the ZnP singlet excited state initially transfers energy to the (phenanthroline)(2)-Cu(I) complex core, producing a metal-to-ligand charge transfer (MLCT) excited state, which in turn transfers an electron to the C(60) group, generating the ZnP-[Cu(phen)(2)](2+)-C(60)(*-) charge-separated state. A further charge shift from the [Cu(phen)(2)](2+) complex to the ZnP subunit, competitive with decay to the ground state, leads to the isoenergetic long distance ZnP(*+)-[Cu(phen)(2)](+)-C(60)(*-) charge-separated radical pair state, which slowly decays back to the ground state on the microsecond time scale. The slow rate of back-electron transfer indicates that in this interlocked system, as in previously studied covalently linked ZnP-C(60) hybrid materials, this process occurs in the Marcus-inverted region.
Figures


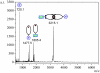
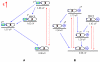
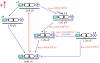
 = Cu(I) ion;
= Cu(I) ion;  =Zn(II)-porphyrin and
=Zn(II)-porphyrin and  = C60.
= C60.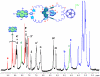


Similar articles
-
Topological and Conformational Effects on Electron Transfer Dynamics in Porphyrin-[60]Fullerene Interlocked Systems.Chem Mater. 2012 Jul 10;24(13):2472-2485. doi: 10.1021/cm3004408. Epub 2012 Jun 18. Chem Mater. 2012. PMID: 22984324 Free PMC article.
-
Energy and electron transfer in beta-alkynyl-linked porphyrin-[60]fullerene dyads.J Phys Chem B. 2006 Jul 27;110(29):14155-66. doi: 10.1021/jp061844t. J Phys Chem B. 2006. PMID: 16854114
-
Azobenzene-linked porphyrin-fullerene dyads.J Am Chem Soc. 2007 Dec 26;129(51):15973-82. doi: 10.1021/ja074684n. Epub 2007 Dec 6. J Am Chem Soc. 2007. PMID: 18052375
-
Synthesis, crystal structure, and photodynamics of π-expanded porphyrin-fullerene dyads synthesized by Diels-Alder reaction.J Phys Chem B. 2010 Nov 18;114(45):14717-28. doi: 10.1021/jp102966x. Epub 2010 Jun 7. J Phys Chem B. 2010. PMID: 20527754
-
Porphyrin-fullerene linked systems as artificial photosynthetic mimics.Org Biomol Chem. 2004 May 21;2(10):1425-33. doi: 10.1039/b403024a. Epub 2004 Apr 26. Org Biomol Chem. 2004. PMID: 15136797 Review.
Cited by
-
Cross dehydrogenative C-O coupling catalysed by a catenane-coordinated copper(i).Chem Sci. 2020 Nov 1;11(48):13008-13014. doi: 10.1039/d0sc05133k. Chem Sci. 2020. PMID: 34094485 Free PMC article.
-
Ru(II)Porphyrinate-based molecular nanoreactor for carbene insertion reactions and quantitative formation of rotaxanes by active-metal-template syntheses.Nat Commun. 2020 Dec 11;11(1):6370. doi: 10.1038/s41467-020-20046-x. Nat Commun. 2020. PMID: 33311502 Free PMC article.
-
Topological and Conformational Effects on Electron Transfer Dynamics in Porphyrin-[60]Fullerene Interlocked Systems.Chem Mater. 2012 Jul 10;24(13):2472-2485. doi: 10.1021/cm3004408. Epub 2012 Jun 18. Chem Mater. 2012. PMID: 22984324 Free PMC article.
-
Multistep energy and electron transfer processes in novel rotaxane donor-acceptor hybrids generating microsecond-lived charge separated states.Chem Sci. 2015 Dec 1;6(12):7293-7304. doi: 10.1039/c5sc02895g. Epub 2015 Oct 2. Chem Sci. 2015. PMID: 28757988 Free PMC article.
-
Quantum-Chemical Insights into the Self-Assembly of Carbon-Based Supramolecular Complexes.Molecules. 2018 Jan 7;23(1):118. doi: 10.3390/molecules23010118. Molecules. 2018. PMID: 29316675 Free PMC article. Review.
References
-
- Cannon RD. Electron Transfer Reactions. Butterworths; London, U. K.: 1980.
- Eberson L. Electron Transfer Reactions in Organic Chemistry. Springer; New York: 1987.
-
- Balzani V, Scandola F. Supramolecular Photochemistry. Horwood; Chichester, U. K.: 1991.
- Wasielewski MR. Chem. Rev. 1992;92:435–461.
-
- Hader DP, Tevini M. General Photobiology. Pergamon; Elmsford, NY: 1987.
- Breton J, Vermeglio H, editors. The Photosynthetic Bacterial Reaction Center. Structure and Dynamics. Plenum; New York: 1988.
- Deisenhofer J, Michel H. Angew. Chem., Int. Ed. Engl. 1989;28:829–847.
- Feher G, Allen JP, Okamura MY, Rees DC. Nature. 1989;339:111–116.
- Moser CC, Keske JM, Warncke K, Farid MS, Duttin PL. Nature. 1992;355:796–802. - PubMed
-
- Imahori H, Sakata Y. Adv. Mat. 1997;9:537–546.
- Echegoyen L, Echegoyen LE. Acc. Chem. Res. 1998;31:593–601.
- Guldi DM. Chem. Comm. 2000;5:321–327.
- Prato M, Guldi DM. Acc. Chem. Res. 2000;33:695–703. - PubMed
- Guldi DM. Chem. Soc. Rev. 2002;31:22–36. - PubMed
- Fukuzumi S, Ohkubo K, Imahori H, Guldi DM. Chem. Eur. J. 2003;9:1585–1593. - PubMed
- Figueira-Duarte T, Lloveras V, Vidal-Gancedo J, Gegout A, Delavaux NB, Welter R, Veciana J, Rovira C, Nierengarten JF. Chem. Commum. 2007;42:4345–4347. - PubMed
- Regehly M, Ermilov EA, Helmreich M, Hirsch A, Jux N, Roeder B. J. Phys. Chem. B. 2007;111:998–1006. - PubMed
- Santos J, Grim B, Islescas BM, Martin N. Chem. Comm. 2008;45:5993–5995. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

