Role for protein kinase C delta in the functional activity of human UGT1A6: implications for drug-drug interactions between PKC inhibitors and UGT1A6
- PMID: 20196639
- PMCID: PMC6170201
- DOI: 10.3109/00498251003596817
Role for protein kinase C delta in the functional activity of human UGT1A6: implications for drug-drug interactions between PKC inhibitors and UGT1A6
Abstract
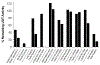
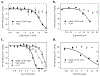
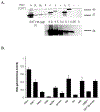
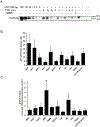
Many UDP-glucuronosyltransferases (UGTs) require phosphorylation by protein kinase C (PKC) for glucuronidation activity. Inhibition of UGT phosphorylation by PKC inhibitor drugs may represent a novel mechanism for drug-drug interactions. The potential for PKC-mediated inhibition of human UGT1A6, an isoform involved in the glucuronidation of drugs such as acetaminophen (paracetamol) and endogenous substrates including serotonin, was evaluated using various cell model systems. Of ten different PKC inhibitors screened for their effects on acetaminophen glucuronidation by human LS180 colon cells, only rottlerin (PKC delta selective inhibitor; IC(50) = 9.0 +/- 1.2 microM) and the non-selective PKC inhibitors (calphostin-C, curcumin and hypericin) decreased glucuronidation by more than 50%. Using UGT1A6-infected Sf9 insect cells, calphostin-C and hypericin showed three times more potent inhibition of serotonin glucuronidation in treated whole cells versus cell lysates. However, both curcumin and rottlerin showed significant direct inhibition and so (indirect) PKC effects could not be differentiated in this model system. Of nine PKC isoforms co-expressed with UGT1A6 in human embryonic kidney 293T cells only PKC delta increased protein-normalized UGT1A6-mediated serotonin glucuronidation significantly (by 63% +/- 4%). These results identify an important role for PKC delta in UGT1A6-mediated glucuronidation and suggest that PKC delta inhibitors could interfere with glucuronidation of UGT1A6 substrates.
Conflict of interest statement
Figures

Similar articles
-
Human liver UDP-glucuronosyltransferase isoforms involved in the glucuronidation of 7-ethyl-10-hydroxycamptothecin.Xenobiotica. 2001 Oct;31(10):687-99. doi: 10.1080/00498250110057341. Xenobiotica. 2001. PMID: 11695848
-
Evaluation of 5-hydroxytryptophol and other endogenous serotonin (5-hydroxytryptamine) analogs as substrates for UDP-glucuronosyltransferase 1A6.Drug Metab Dispos. 2004 Aug;32(8):862-9. doi: 10.1124/dmd.32.8.862. Drug Metab Dispos. 2004. PMID: 15258112
-
Validation of serotonin (5-hydroxtryptamine) as an in vitro substrate probe for human UDP-glucuronosyltransferase (UGT) 1A6.Drug Metab Dispos. 2003 Jan;31(1):133-9. doi: 10.1124/dmd.31.1.133. Drug Metab Dispos. 2003. PMID: 12485962
-
UDP-glucuronosyltransferase 1A6: structural, functional, and regulatory aspects.Methods Enzymol. 2005;400:57-75. doi: 10.1016/S0076-6879(05)00004-2. Methods Enzymol. 2005. PMID: 16399343 Review.
-
Isoform-selective probe substrates for in vitro studies of human UDP-glucuronosyltransferases.Methods Enzymol. 2005;400:104-16. doi: 10.1016/S0076-6879(05)00007-8. Methods Enzymol. 2005. PMID: 16399346 Review.
Cited by
-
Potential Herb-Drug Interactions in the Management of Age-Related Cognitive Dysfunction.Pharmaceutics. 2021 Jan 19;13(1):124. doi: 10.3390/pharmaceutics13010124. Pharmaceutics. 2021. PMID: 33478035 Free PMC article. Review.
-
Regulated phosphorylation of a major UDP-glucuronosyltransferase isozyme by tyrosine kinases dictates endogenous substrate selection for detoxification.J Biol Chem. 2011 Jan 14;286(2):1639-48. doi: 10.1074/jbc.M110.165126. Epub 2010 Nov 5. J Biol Chem. 2011. PMID: 21056984 Free PMC article.
-
First-pass metabolism via UDP-glucuronosyltransferase: a barrier to oral bioavailability of phenolics.J Pharm Sci. 2011 Sep;100(9):3655-81. doi: 10.1002/jps.22568. Epub 2011 Apr 11. J Pharm Sci. 2011. PMID: 21484808 Free PMC article. Review.
-
Interaction between different extracts of Hypericum perforatum L. from Serbia and pentobarbital, diazepam and paracetamol.Molecules. 2014 Mar 28;19(4):3869-82. doi: 10.3390/molecules19043869. Molecules. 2014. PMID: 24686576 Free PMC article.
-
Homology Modeling of Human Uridine-5'-diphosphate-glucuronosyltransferase 1A6 Reveals Insights into Factors Influencing Substrate and Cosubstrate Binding.ACS Omega. 2020 Mar 20;5(12):6872-6887. doi: 10.1021/acsomega.0c00205. eCollection 2020 Mar 31. ACS Omega. 2020. PMID: 32258923 Free PMC article.
References
-
- Abraham C, Scaglione-Sewell B, Skarosi SF, Qin W, Bissonnette M, Brasitus TA. (1998). Protein kinase C alpha modulates growth and differentiation in Caco-2 cells. Gastroenterology 114:503–509. - PubMed
-
- Assert R, Kotter R, Bisping G, Scheppach W, Stahlnecker E, Muller KM, Dusel G, Schatz H, Pfeiffer A. (1999). Anti-proliferative activity of protein kinase C in apical compartments of human colonic crypts: evidence for a less activated protein kinase C in small adenomas. Int J Cancer 80:47–53. - PubMed
-
- Basu NK, Kole L, Owens IS. (2003). Evidence for phosphorylation requirement for human bilirubin UDP-glucuronosyltransferase (UGT1A1) activity. Biochem Biophys Res Commun 303:98–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
