Altered SDF-1-mediated differentiation of bone marrow-derived endothelial progenitor cells in diabetes mellitus
- PMID: 20196780
- PMCID: PMC4516496
- DOI: 10.1111/j.1582-4934.2009.00655.x
Altered SDF-1-mediated differentiation of bone marrow-derived endothelial progenitor cells in diabetes mellitus
Abstract
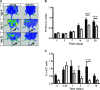
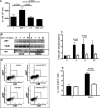
In diabetic patients and animal models of diabetes mellitus (DM), circulating endothelial progenitor cell (EPC) number is lower than in normoglycaemic conditions and EPC angiogenic properties are inhibited. Stromal cell derived factor-1 (SDF-1) plays a key role in bone marrow (BM) c-kit(+) stem cell mobilization into peripheral blood (PB), recruitment from PB into ischemic tissues and differentiation into endothelial cells. The aim of the present study was to examine the effect of DM in vivo and in vitro, on murine BM-derived c-kit(+) cells and on their response to SDF-1. Acute hindlimb ischemia was induced in streptozotocin-treated DM and control mice; circulating c-kit(+) cells exhibited a rapid increase followed by a return to control levels which was significantly faster in DM than in control mice. CXCR4 expression by BM c-kit(+) cells as well as SDF-1 protein levels in the plasma and in the skeletal muscle, both before and after the induction of ischemia, were similar between normoglycaemic and DM mice. However, BM-derived c-kit(+) cells from DM mice exhibited an impaired differentiation towards the endothelial phenotype in response to SDF-1; this effect was associated with diminished protein kinase phosphorylation. Interestingly, SDF-1 ability to induce differentiation of c-kit(+) cells from DM mice was restored when cells were cultured under normoglycaemic conditions whereas c-kit(+) cells from normoglycaemic mice failed to differentiate in response to SDF-1 when they were cultured in hyperglycaemic conditions. These results show that DM diminishes circulating c-kit(+) cell number following hindlimb ischemia and inhibits SDF-1-mediated AKT phosphorylation and differentiation towards the endothelial phenotype of BM-derived c-kit(+) cells.
Figures


Similar articles
-
SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells.Blood. 2004 Dec 1;104(12):3472-82. doi: 10.1182/blood-2003-12-4423. Epub 2004 Jul 29. Blood. 2004. PMID: 15284120
-
Role of nox2-based NADPH oxidase in bone marrow and progenitor cell function involved in neovascularization induced by hindlimb ischemia.Circ Res. 2008 Jul 18;103(2):212-20. doi: 10.1161/CIRCRESAHA.108.176230. Epub 2008 Jun 26. Circ Res. 2008. PMID: 18583711 Free PMC article.
-
A critical role of Src family kinase in SDF-1/CXCR4-mediated bone-marrow progenitor cell recruitment to the ischemic heart.J Mol Cell Cardiol. 2015 Apr;81:49-53. doi: 10.1016/j.yjmcc.2015.01.024. Epub 2015 Feb 3. J Mol Cell Cardiol. 2015. PMID: 25655934 Free PMC article.
-
Progenitor cell mobilization and recruitment: SDF-1, CXCR4, α4-integrin, and c-kit.Prog Mol Biol Transl Sci. 2012;111:243-64. doi: 10.1016/B978-0-12-398459-3.00011-3. Prog Mol Biol Transl Sci. 2012. PMID: 22917234 Free PMC article. Review.
-
Hyperoxia, endothelial progenitor cell mobilization, and diabetic wound healing.Antioxid Redox Signal. 2008 Nov;10(11):1869-82. doi: 10.1089/ars.2008.2121. Antioxid Redox Signal. 2008. PMID: 18627349 Free PMC article. Review.
Cited by
-
MicroRNA-27b Impairs Nrf2-Mediated Angiogenesis in the Progression of Diabetic Foot Ulcer.J Clin Med. 2023 Jul 7;12(13):4551. doi: 10.3390/jcm12134551. J Clin Med. 2023. PMID: 37445586 Free PMC article.
-
Identification of novel diabetes impaired miRNA-transcription factor co-regulatory networks in bone marrow-derived Lin-/VEGF-R2+ endothelial progenitor cells.PLoS One. 2018 Jul 11;13(7):e0200194. doi: 10.1371/journal.pone.0200194. eCollection 2018. PLoS One. 2018. PMID: 29995913 Free PMC article.
-
Pharmacological restoration of autophagy reduces hypertension-related stroke occurrence.Autophagy. 2020 Aug;16(8):1468-1481. doi: 10.1080/15548627.2019.1687215. Epub 2019 Nov 12. Autophagy. 2020. PMID: 31679456 Free PMC article.
-
SDF-1-edited human amniotic mesenchymal stem cells stimulate angiogenesis in treating hindlimb ischaemia.J Cell Mol Med. 2022 Jul;26(13):3726-3735. doi: 10.1111/jcmm.17401. Epub 2022 May 26. J Cell Mol Med. 2022. PMID: 35615995 Free PMC article.
-
Molecular mechanisms associated with diabetic endothelial-erectile dysfunction.Nat Rev Urol. 2016 May;13(5):266-74. doi: 10.1038/nrurol.2016.23. Epub 2016 Feb 16. Nat Rev Urol. 2016. PMID: 26878803 Review.
References
-
- Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. - PubMed
-
- Takahashi T, Kalka C, Masuda H, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–8. - PubMed
-
- Asahara T, Masuda H, Takahashi T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–8. - PubMed
-
- Tateishi-Yuyama E, Matsubara H, Murohara T, et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360:427. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

