Interferon regulatory factor-8 modulates the development of tumour-induced CD11b+Gr-1+ myeloid cells
- PMID: 20196788
- PMCID: PMC3858838
- DOI: 10.1111/j.1582-4934.2009.00685.x
Interferon regulatory factor-8 modulates the development of tumour-induced CD11b+Gr-1+ myeloid cells
Abstract
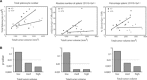
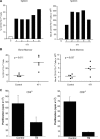
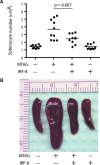
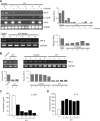
Tumour-induced myeloid-derived suppressor cells (MDSC) promote immune suppression and mediate tumour progression. However, the molecular basis for the generation of MDSC, which in mice co-express the CD11b(+) and Gr-1(+) cell surface markers remains unclear. Because CD11b(+)Gr-1(+) cells expand during progressive tumour growth, this suggests that tumour-induced events alter signalling pathways that affect normal myeloid cell development. Interferon regulatory factor-8 (IRF-8), a member of the IFN-gamma regulatory factor family, is essential for normal myelopoiesis. We therefore examined whether IRF-8 modulated tumour-induced CD11b(+)Gr-1(+) cell development or accumulation using both implantable (4T1) and transgenic (MMTV-PyMT) mouse models of mammary tumour growth. In the 4T1 model, both splenic and bone marrow-derived CD11b(+)Gr-1(+) cells of tumour-bearing mice displayed a marked reduction in IRF-8 expression compared to control populations. A causal link between IRF-8 expression and the emergence of tumour-induced CD11b(+)Gr-1(+) cells was explored in vivo using a double transgenic (dTg) mouse model designed to express transgenes for both IRF-8 and mammary carcinoma development. Despite the fact that tumour growth was unaffected, splenomegaly, as well as the frequencies and absolute numbers of CD11b(+)Gr-1(+) cells were significantly lower in dTg mice when compared with single transgenic tumour-bearing mice. Overall, these data reveal that IRF-8 plays an important role in tumour-induced development and/or accumulation of CD11b(+)Gr-1(+) cells, and establishes a molecular basis for the potential manipulation of these myeloid populations for cancer therapy.
Figures


Similar articles
-
Modulating the expression of IFN regulatory factor 8 alters the protumorigenic behavior of CD11b+Gr-1+ myeloid cells.J Immunol. 2009 Jul 1;183(1):117-28. doi: 10.4049/jimmunol.0804132. J Immunol. 2009. PMID: 19542426 Free PMC article.
-
Premetastatic soil and prevention of breast cancer brain metastasis.Neuro Oncol. 2013 Jul;15(7):891-903. doi: 10.1093/neuonc/not031. Epub 2013 Apr 17. Neuro Oncol. 2013. PMID: 23595625 Free PMC article.
-
IL-33 expands suppressive CD11b+ Gr-1(int) and regulatory T cells, including ST2L+ Foxp3+ cells, and mediates regulatory T cell-dependent promotion of cardiac allograft survival.J Immunol. 2011 Nov 1;187(9):4598-610. doi: 10.4049/jimmunol.1100519. Epub 2011 Sep 26. J Immunol. 2011. PMID: 21949025 Free PMC article.
-
Relevance of Interferon Regulatory Factor-8 Expression in Myeloid-Tumor Interactions.J Interferon Cytokine Res. 2016 Jul;36(7):442-53. doi: 10.1089/jir.2015.0174. J Interferon Cytokine Res. 2016. PMID: 27379866 Free PMC article. Review.
-
Subsets, expansion and activation of myeloid-derived suppressor cells.Med Microbiol Immunol. 2010 Aug;199(3):273-81. doi: 10.1007/s00430-010-0151-4. Epub 2010 Apr 8. Med Microbiol Immunol. 2010. PMID: 20376485 Review.
Cited by
-
Baseline Splenic Volume as a Prognostic Biomarker of FOLFIRI Efficacy and a Surrogate Marker of MDSC Accumulation in Metastatic Colorectal Carcinoma.Cancers (Basel). 2020 May 31;12(6):1429. doi: 10.3390/cancers12061429. Cancers (Basel). 2020. PMID: 32486421 Free PMC article.
-
Transcriptional regulation of myeloid-derived suppressor cells.J Leukoc Biol. 2015 Dec;98(6):913-22. doi: 10.1189/jlb.4RI0515-204R. Epub 2015 Sep 3. J Leukoc Biol. 2015. PMID: 26337512 Free PMC article. Review.
-
Selective blockade of B7-H3 enhances antitumour immune activity by reducing immature myeloid cells in head and neck squamous cell carcinoma.J Cell Mol Med. 2017 Sep;21(9):2199-2210. doi: 10.1111/jcmm.13143. Epub 2017 Apr 11. J Cell Mol Med. 2017. PMID: 28401653 Free PMC article.
-
Sensitivity of a novel model of mammary cancer stem cell-like cells to TNF-related death pathways.Cancer Immunol Immunother. 2012 Aug;61(8):1255-68. doi: 10.1007/s00262-012-1200-1. Epub 2012 Jan 24. Cancer Immunol Immunother. 2012. PMID: 22270714 Free PMC article.
-
Deregulation of apoptotic factors Bcl-xL and Bax confers apoptotic resistance to myeloid-derived suppressor cells and contributes to their persistence in cancer.J Biol Chem. 2013 Jun 28;288(26):19103-15. doi: 10.1074/jbc.M112.434530. Epub 2013 May 15. J Biol Chem. 2013. PMID: 23677993 Free PMC article.
References
-
- Wang T, Niu G, Kortylewski M, et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nature Med. 2004;10:48–54. - PubMed
-
- Stewart TJ, Greeneltch KM, Lutsiak ME, et al. Immunological responses can have both pro- and antitumour effects: implications for immunotherapy. Expert Rev Mol Med. 2007;9:1–20. - PubMed
-
- Nagaraj S, Gabrilovich DI. Myeloid-derived suppressor cells. Adv Exp Med Biol. 2007;601:213–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

