The loss of health status in rheumatoid arthritis and the effect of biologic therapy: a longitudinal observational study
- PMID: 20196859
- PMCID: PMC2888182
- DOI: 10.1186/ar2944
The loss of health status in rheumatoid arthritis and the effect of biologic therapy: a longitudinal observational study
Abstract
Introduction: The long-term course of rheumatoid arthritis (RA) in terms of health status is not well understood, nor is the degree of effectiveness of biologic therapy in the community. We modeled the progression of loss of health status, and measured incremental costs and effectiveness of biologic therapy in the community.
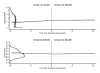
Methods: We studied change in function and health status in 18,485 RA patients (135,731 observations) at six-month intervals for up to 11 years, including a group of 4,911 patients (59,630 observations) who switched to biologic therapy from non-biologic therapy. We measured the SF-36 Physical Component (PCS) and Mental Component (MCS) Summary scales, the EQ-5D health utility scale, and the Health Assessment Questionnaire (HAQ) disability scale; and we calculated treatment and direct medical costs.
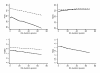
Results: RA onset caused an immediate and substantial reduction in physical but not mental health status. Thereafter, the progression of dysfunction in RA was very slow (HAQ 0.016 units and PCS -0.125 units annually), only slightly worse than the age and sex-adjusted US population. We estimated biologic treatment to improve HAQ by 0.29 units, PCS by 5.3 units, and EQ-5D by 0.05 units over a 10-year period. The estimated incremental 10-year total direct medical cost for this benefit was $159,140.
Conclusions: Biologic therapy retards RA progression, but its effect is far less than is seen in clinical trials. In the community, cost-effectiveness is substantially less than that estimated from clinical trial data. The study results represent the incremental benefit of adding biologic therapy to optimum non-biologic therapy.
Figures
Comment in
-
Clinical effectiveness of biologics in clinical practice.Arthritis Res Ther. 2010;12(2):115. doi: 10.1186/ar2970. Epub 2010 Apr 28. Arthritis Res Ther. 2010. PMID: 20447320 Free PMC article.
References
-
- Klareskog L, Heijde D van der, de Jager JP, Gough A, Kalden J, Malaise M, Martín Mola E, Pavelka K, Sany J, Settas L, Wajdula J, Pedersen R, Fatenejad S, Sanda M. TEMPO (Trial of Etanercept and Methotrexate with Radiographic Patient Outcomes) study investigators. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004;363:675–681. doi: 10.1016/S0140-6736(04)15640-7. - DOI - PubMed
-
- Breedveld FC, Weisman MH, Kavanaugh AF, Cohen SB, Pavelka K, Van Vollenhoven R, Sharp J, Perez JL, Spencer-Green GT. The PREMIER study: A multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26–37. doi: 10.1002/art.21519. - DOI - PubMed
-
- Putte LB van de, Atkins C, Malaise M, Sany J, Russell AS, van Riel PL, Settas L, Bijlsma JW, Todesco S, Dougados M, Nash P, Emery P, Walter N, Kaul M, Fischkoff S, Kupper H. Efficacy and safety of adalimumab as monotherapy in patients with rheumatoid arthritis for whom previous disease modifying antirheumatic drug treatment has failed. Ann Rheum Dis. 2004;63:508–516. doi: 10.1136/ard.2003.013052. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

