An iris-like mechanism of pore dilation in the CorA magnesium transport system
- PMID: 20197031
- PMCID: PMC2830438
- DOI: 10.1016/j.bpj.2009.11.009
An iris-like mechanism of pore dilation in the CorA magnesium transport system
Abstract
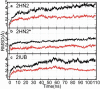
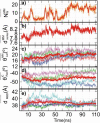
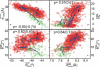
Magnesium translocation across cell membranes is essential for numerous physiological processes. Three recently reported crystal structures of the CorA magnesium transport system revealed a surprising architecture, with a bundle of giant alpha-helices forming a 60-A-long pore that extends beyond the membrane before widening into a funnel-shaped cytosolic domain. The presence of divalent cations in putative intracellular regulation sites suggests that these structures correspond to the closed conformation of CorA. To examine the nature of the conduction pathway, we performed 110-ns molecular-dynamics simulations of two of these structures in a lipid bilayer with and without regulatory ions. The results show that a 15-A-long hydrophobic constriction straddling the membrane-cytosol interface constitutes a steric bottleneck whose location coincides with an electrostatic barrier opposing cation translocation. In one of the simulations, structural relaxation after the removal of regulatory ions led to concerted changes in the tilt of the pore helices, resulting in iris-like dilation and spontaneous hydration of the hydrophobic neck. This simple and robust mechanism is consistent with the regulation of pore opening by intracellular magnesium concentration, and explains the unusual architecture of CorA.
2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
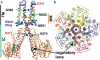



References
-
- Touyz R.M. Magnesium and hypertension. Curr. Opin. Nephrol. Hypertens. 2006;15:141–144. - PubMed
-
- Maguire M.E., Cowan J.A. Mg2+ chemistry and biochemistry. Biometals. 2002;15:203–210. - PubMed
-
- Whelton P.K., Klag M.J. Magnesium and blood pressure: review of the epidemiologic and clinical trial experience. Am. J. Cardiol. 1989;63:26G–30G. - PubMed
-
- Eshaghi S., Niegowski D., Nordlund P. Crystal structure of a divalent metal ion transporter CorA at 2.9 Ångstrom resolution. Science. 2006;313:354–357. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

