The epidermal Ca(2+) gradient: Measurement using the phasor representation of fluorescent lifetime imaging
- PMID: 20197045
- PMCID: PMC2830439
- DOI: 10.1016/j.bpj.2009.10.055
The epidermal Ca(2+) gradient: Measurement using the phasor representation of fluorescent lifetime imaging
Abstract
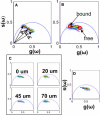
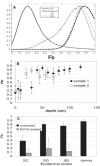
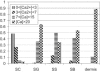
Ionic gradients are found across a variety of tissues and organs. In this report, we apply the phasor representation of fluorescence lifetime imaging data to the quantitative study of ionic concentrations in tissues, overcoming technical problems of tissue thickness, concentration artifacts of ion-sensitive dyes, and calibration across inhomogeneous tissue. We used epidermis as a model system, as Ca(2+) gradients in this organ have been shown previously to control essential biologic processes of differentiation and formation of the epidermal permeability barrier. The approach described here allowed much better localization of Ca(2+) stores than those used in previous studies, and revealed that the bulk of free Ca(2+) measured in the epidermis comes from intracellular Ca(2+) stores such as the Golgi and the endoplasmic reticulum, with extracellular Ca(2+) making a relatively small contribution to the epidermal Ca(2+) gradient. Due to the high spatial resolution of two-photon microscopy, we were able to measure a marked heterogeneity in average calcium concentrations from cell to cell in the basal keratinocytes. This finding, not reported in previous studies, calls into question the long-held hypothesis that keratinocytes increase intracellular Ca(2+), cease proliferation, and differentiate passively in response to changes in extracellular Ca(2+). The experimental results obtained using this approach illustrate the power of the experimental and analytical techniques outlined in this report. Our approach can be used in mechanistic studies to address the formation, maintenance, and function of the epidermal Ca(2+) gradient, and it should be broadly applicable to the study of other tissues with ionic gradients.
2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Skin Barrier and Calcium.Ann Dermatol. 2018 Jun;30(3):265-275. doi: 10.5021/ad.2018.30.3.265. Epub 2018 Apr 23. Ann Dermatol. 2018. PMID: 29853739 Free PMC article. Review.
-
Major translocation of calcium upon epidermal barrier insult: imaging and quantification via FLIM/Fourier vector analysis.Arch Dermatol Res. 2011 Mar;303(2):103-15. doi: 10.1007/s00403-010-1113-9. Epub 2010 Dec 31. Arch Dermatol Res. 2011. PMID: 21193994 Free PMC article.
-
Selective obliteration of the epidermal calcium gradient leads to enhanced lamellar body secretion.J Invest Dermatol. 1994 May;102(5):789-95. doi: 10.1111/1523-1747.ep12377921. J Invest Dermatol. 1994. PMID: 8176264
-
Epidermal expression of the full-length extracellular calcium-sensing receptor is required for normal keratinocyte differentiation.J Cell Physiol. 2002 Jul;192(1):45-54. doi: 10.1002/jcp.10107. J Cell Physiol. 2002. PMID: 12115735
-
The role of the calcium-sensing receptor in epidermal differentiation.Cell Calcium. 2004 Mar;35(3):265-73. doi: 10.1016/j.ceca.2003.10.019. Cell Calcium. 2004. PMID: 15200150 Review.
Cited by
-
Regulation of ERK-MAPK signaling in human epidermis.BMC Syst Biol. 2015 Jul 25;9:41. doi: 10.1186/s12918-015-0187-6. BMC Syst Biol. 2015. PMID: 26209520 Free PMC article.
-
Activation of transient receptor potential vanilloid 3 is required for keratinocyte differentiation and epidermal barrier formation.Korean J Physiol Pharmacol. 2025 Jul 1;29(4):409-418. doi: 10.4196/kjpp.24.324. Epub 2025 Apr 28. Korean J Physiol Pharmacol. 2025. PMID: 40288993 Free PMC article.
-
Hailey-Hailey Disease Successfully Treated With Adalimumab: A Case Series.Cureus. 2024 Aug 19;16(8):e67227. doi: 10.7759/cureus.67227. eCollection 2024 Aug. Cureus. 2024. PMID: 39295647 Free PMC article.
-
Skin Barrier and Calcium.Ann Dermatol. 2018 Jun;30(3):265-275. doi: 10.5021/ad.2018.30.3.265. Epub 2018 Apr 23. Ann Dermatol. 2018. PMID: 29853739 Free PMC article. Review.
-
Calcium regulation of stem cells.EMBO Rep. 2020 Jun 4;21(6):e50028. doi: 10.15252/embr.202050028. Epub 2020 May 17. EMBO Rep. 2020. PMID: 32419314 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

