MEF-2 regulates activity-dependent spine loss in striatopallidal medium spiny neurons
- PMID: 20197093
- PMCID: PMC2878643
- DOI: 10.1016/j.mcn.2010.01.012
MEF-2 regulates activity-dependent spine loss in striatopallidal medium spiny neurons
Abstract
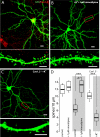
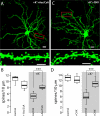
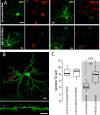
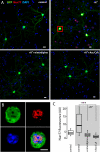
Striatal dopamine depletion profoundly reduces the density of spines and corticostriatal glutamatergic synapses formed on D(2) dopamine receptor expressing striatopallidal medium spiny neurons, leaving D(1) receptor expressing striatonigral medium spiny neurons relatively intact. Because D(2) dopamine receptors diminish the excitability of striatopallidal MSNs, the pruning of synapses could be a form of homeostatic plasticity aimed at restoring activity into a preferred range. To characterize the homeostatic mechanisms controlling synapse density in striatal medium spiny neurons, striatum from transgenic mice expressing a D(2) receptor reporter construct was co-cultured with wild-type cerebral cortex. Sustained depolarization of these co-cultures induced a profound pruning of glutamatergic synapses and spines in striatopallidal medium spiny neurons. This pruning was dependent upon Ca(2+) entry through Cav1.2 L-type Ca(2+) channels, activation of the Ca(2+)-dependent protein phosphatase calcineurin and up-regulation of myocyte enhancer factor 2 (MEF2) transcriptional activity. Depolarization and MEF2 up-regulation increased the expression of two genes linked to synaptic remodeling-Nur77 and Arc. Taken together, these studies establish a translational framework within which striatal adaptations linked to the symptoms of Parkinson's disease can be explored.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures
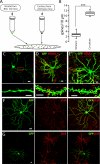
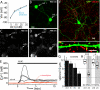
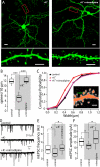
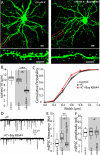
Similar articles
-
Homeostatic regulation of excitatory synapses on striatal medium spiny neurons expressing the D2 dopamine receptor.Brain Struct Funct. 2016 May;221(4):2093-107. doi: 10.1007/s00429-015-1029-4. Epub 2015 Mar 18. Brain Struct Funct. 2016. PMID: 25782435
-
Differential excitability and modulation of striatal medium spiny neuron dendrites.J Neurosci. 2008 Nov 5;28(45):11603-14. doi: 10.1523/JNEUROSCI.1840-08.2008. J Neurosci. 2008. PMID: 18987196 Free PMC article.
-
Differential dopaminergic modulation of neostriatal synaptic connections of striatopallidal axon collaterals.J Neurosci. 2009 Jul 15;29(28):8977-90. doi: 10.1523/JNEUROSCI.6145-08.2009. J Neurosci. 2009. PMID: 19605635 Free PMC article.
-
Differential striatal spine pathology in Parkinson's disease and cocaine addiction: a key role of dopamine?Neuroscience. 2013 Oct 22;251:2-20. doi: 10.1016/j.neuroscience.2013.07.011. Epub 2013 Jul 16. Neuroscience. 2013. PMID: 23867772 Free PMC article. Review.
-
Integrating neurotransmission in striatal medium spiny neurons.Adv Exp Med Biol. 2012;970:407-29. doi: 10.1007/978-3-7091-0932-8_18. Adv Exp Med Biol. 2012. PMID: 22351066 Review.
Cited by
-
Glutamatergic Neurons in the Piriform Cortex Influence the Activity of D1- and D2-Type Receptor-Expressing Olfactory Tubercle Neurons.J Neurosci. 2019 Nov 27;39(48):9546-9559. doi: 10.1523/JNEUROSCI.1444-19.2019. Epub 2019 Oct 18. J Neurosci. 2019. PMID: 31628176 Free PMC article.
-
Emerging roles for MEF2 in brain development and mental disorders.Curr Opin Neurobiol. 2019 Dec;59:49-58. doi: 10.1016/j.conb.2019.04.008. Epub 2019 May 23. Curr Opin Neurobiol. 2019. PMID: 31129473 Free PMC article. Review.
-
Single-nucleus analysis of accessible chromatin in developing mouse forebrain reveals cell-type-specific transcriptional regulation.Nat Neurosci. 2018 Mar;21(3):432-439. doi: 10.1038/s41593-018-0079-3. Epub 2018 Feb 12. Nat Neurosci. 2018. PMID: 29434377 Free PMC article.
-
A role for dendritic mGluR5-mediated local translation of Arc/Arg3.1 in MEF2-dependent synapse elimination.Cell Rep. 2014 Jun 12;7(5):1589-1600. doi: 10.1016/j.celrep.2014.04.035. Epub 2014 May 22. Cell Rep. 2014. PMID: 24857654 Free PMC article.
-
MEF2 transcription factors: developmental regulators and emerging cancer genes.Oncotarget. 2016 Jan 19;7(3):2297-312. doi: 10.18632/oncotarget.6223. Oncotarget. 2016. PMID: 26506234 Free PMC article. Review.
References
-
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends in neurosciences. 1989;12:366–375. - PubMed
-
- Bevan MD, Magill PJ, Terman D, Bolam JP, Wilson CJ. Move to the rhythm: oscillations in the subthalamic nucleus-external globus pallidus network. Trends in neurosciences. 2002;25:525–531. - PubMed
-
- Blackstone C, Sheng M. Protein targeting and calcium signaling microdomains in neuronal cells. Cell Calcium. 1999;26:181–192. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

