Epicardial neural ganglionated plexus of ovine heart: anatomic basis for experimental cardiac electrophysiology and nerve protective cardiac surgery
- PMID: 20197118
- PMCID: PMC3025183
- DOI: 10.1016/j.hrthm.2010.02.036
Epicardial neural ganglionated plexus of ovine heart: anatomic basis for experimental cardiac electrophysiology and nerve protective cardiac surgery
Abstract
Background: Sheep are routinely used in experimental cardiac electrophysiology and surgery.
Objective: The purpose of this study was to (1) ascertain the topography and architecture of the ovine epicardial neural plexus (ENP), (2) determine the relationships of ENP with vagal and sympathetic cardiac nerves and ganglia, and (3) evaluate gross anatomic differences and similarities of ENP in humans, sheep, and other species.
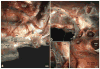
Methods: Ovine ENP and extrinsic sympathetic and vagal nerves were stained histochemically for acetylcholinesterase in whole heart and/or thorax-dissected preparations from 23 newborn lambs, with subsequent examination by stereomicroscope.
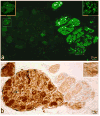
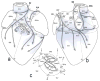
Results: Intrinsic cardiac nerves extend from the venous part of the ovine heart hilum along the roots of the cranial (superior) caval and left azygos veins to both atria and ventricles via five epicardial routes: dorsal right atrial, middle dorsal, left dorsal, right ventral, and ventral left atrial nerve subplexuses. Intrinsic nerves proceeding from the arterial part of the heart hilum along the roots of the aorta and pulmonary trunk extend exclusively into the ventricles as the right and left coronary subplexuses. The dorsal right atrial, right ventral, and middle dorsal subplexuses receive the main extrinsic neural input from the right cervicothoracic and right thoracic sympathetic T(2) and T(3) ganglia as well as from the right vagal nerve. The left dorsal is supplied by sizeable extrinsic nerves from the left thoracic T(4)-T(6) sympathetic ganglia and the left vagal nerve. Sheep hearts contained an average of 769 +/- 52 epicardial ganglia. Cumulative areas of epicardial ganglia on the root of the cranial vena cava and on the wall of the coronary sinus were the largest of all regions (P <.05).
Conclusion: Despite substantial interindividual variability in the morphology of ovine ENP, right-sided epicardial neural subplexuses supplying the sinoatrial and atrioventricular nodes are mostly concentrated at a fat pad between the right pulmonary veins and the cranial vena cava. This finding is in sharp contrast with a solely left lateral neural input to the human atrioventricular node, which extends mainly from the left dorsal and middle dorsal subplexuses. The abundance of epicardial ganglia distributed widely along the ovine ventricular nerves over respectable distances below the coronary groove implies a distinctive neural control of the ventricles in human and sheep hearts.
Copyright 2010 Heart Rhythm Society. All rights reserved.
Conflict of interest statement
No Conflicts
Figures




Comment in
-
Remembrances of time past, or A la recherche du temps perdu.Heart Rhythm. 2010 Jul;7(7):951-2. doi: 10.1016/j.hrthm.2010.03.009. Epub 2010 Mar 10. Heart Rhythm. 2010. PMID: 20226276 No abstract available.
-
Highlights in basic autonomic neurosciences: The vagus and the ventricles.Auton Neurosci. 2012 Sep 25;170(1-2):1-2. doi: 10.1016/j.autneu.2012.07.006. Epub 2012 Aug 14. Auton Neurosci. 2012. PMID: 22902010 No abstract available.
References
-
- Gorman MW, Tune JD, Richmond KN, Feigl EO. Quantitative analysis of feedforward sympathetic coronary vasodilation in exercising dogs. J Appl Physiol. 2000;89:1903–1911. - PubMed
-
- Tsuboi M, Furukawa Y, Nakajima K, Kurogouchi F, Chiba S. Inotropic, chronotropic, and dromotropic effects mediated via parasympathetic ganglia in the dog heart. Am J Physiol Heart Circ Physiol. 2000;279:H1201–1207. - PubMed
-
- Cifelli C, Rose RA, Zhang H, et al. RGS4 regulates parasympathetic signaling and heart rate control in the sinoatrial node. Circ Res. 2008;103:527–535. - PubMed
-
- Levy MN, Warner MR. Parasympathetic effects on cardiac function. In: Armour JA, Ardell JL, editors. Neurocardiology. New York: Oxford University Press; 1994. pp. 53–76.
-
- Armour JA, Kember GC. Cardiac sensory neurons. In: Armour JA, Ardell JL, editors. Basic and clinical neurocardiology. New York: Oxford University Press; 2004. pp. 79–117.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

