Rotavirus enterotoxin NSP4 has mucosal adjuvant properties
- PMID: 20197138
- PMCID: PMC3663485
- DOI: 10.1016/j.vaccine.2010.02.063
Rotavirus enterotoxin NSP4 has mucosal adjuvant properties
Abstract
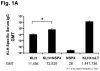
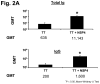
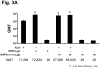
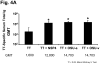
Rotavirus nonstructural protein 4 (NSP4) is a protein with pleiotropic properties. It functions in rotavirus morphogenesis, pathogenesis, and is the first described viral enterotoxin. Since many bacterial toxins function as potent mucosal adjuvants, we evaluated whether baculovirus-expressed recombinant simian rotavirus SA11 NSP4 possesses adjuvant activity by co-administering NSP4 with keyhole limpet hemocyanin (KLH), tetanus toxoid (TT) or ovalbumin (OVA) as model antigens in mice. Following intranasal immunization, NSP4 significantly enhanced both systemic and mucosal immune responses to model immunogens, as compared to the control group, in an antigen-specific manner. Both full-length and a cleavage product of SA11 NSP4 had adjuvant activity, localizing this activity to the C-terminus of the protein. NSP4 forms from virulent and avirulent porcine rotavirus OSU strain, and SA11 NSP4 localized within a 2/6-virus-like particle (VLP) also exhibited adjuvant effects. These studies suggest that the rotavirus enterotoxin NSP4 can function as an adjuvant to enhance immune responses for a co-administered antigen.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures







Similar articles
-
Immunization of Mice by Rotavirus NSP4-VP6 Fusion Protein Elicited Stronger Responses Compared to VP6 Alone.Viral Immunol. 2018 Apr;31(3):233-241. doi: 10.1089/vim.2017.0075. Epub 2017 Nov 29. Viral Immunol. 2018. PMID: 29185875
-
Mucosal immunization with a ricin toxin B subunit-rotavirus NSP4 fusion protein stimulates a Th1 lymphocyte response.J Biotechnol. 2006 Jan 24;121(2):272-83. doi: 10.1016/j.jbiotec.2005.07.024. Epub 2005 Sep 21. J Biotechnol. 2006. PMID: 16181698
-
Oral immunization with a shiga toxin B subunit: rotavirus NSP4(90) fusion protein protects mice against gastroenteritis.Vaccine. 2005 Oct 25;23(44):5168-76. doi: 10.1016/j.vaccine.2005.06.015. Vaccine. 2005. PMID: 16040169
-
Dendritic cell-targeting DNA-based nasal adjuvants for protective mucosal immunity to Streptococcus pneumoniae.Microbiol Immunol. 2017 Jun;61(6):195-205. doi: 10.1111/1348-0421.12487. Microbiol Immunol. 2017. PMID: 28463465 Free PMC article. Review.
-
Rotavirus protein expression is important for virus assembly and pathogenesis.Arch Virol Suppl. 1996;12:69-77. doi: 10.1007/978-3-7091-6553-9_8. Arch Virol Suppl. 1996. PMID: 9015103 Review.
Cited by
-
Oral Administration of a Seed-based Bivalent Rotavirus Vaccine Containing VP6 and NSP4 Induces Specific Immune Responses in Mice.Front Plant Sci. 2017 May 31;8:910. doi: 10.3389/fpls.2017.00910. eCollection 2017. Front Plant Sci. 2017. PMID: 28620404 Free PMC article.
-
Effects of rotavirus NSP4 protein on the immune response and protection of the SR69A-VP8* nanoparticle rotavirus vaccine.Vaccine. 2021 Jan 8;39(2):263-271. doi: 10.1016/j.vaccine.2020.12.005. Epub 2020 Dec 11. Vaccine. 2021. PMID: 33309483 Free PMC article.
-
Effects of Rotavirus NSP4 on the Immune Response and Protection of Rotavirus-Norovirus Recombinant Subunit Vaccines in Different Immune Pathways.Vaccines (Basel). 2024 Sep 8;12(9):1025. doi: 10.3390/vaccines12091025. Vaccines (Basel). 2024. PMID: 39340055 Free PMC article.
-
HSV-1 amplicon vectors launch the production of heterologous rotavirus-like particles and induce rotavirus-specific immune responses in mice.Mol Ther. 2012 Sep;20(9):1810-1820. doi: 10.1038/mt.2012.108. Epub 2012 Jun 19. Mol Ther. 2012. PMID: 22713696 Free PMC article.
-
Designing, Construction and Expression of a Recombinant Fusion Protein Comprising the Hepatitis E Virus ORF2 and Rotavirus NSP4 in the Baculovirus Expression System.Jundishapur J Microbiol. 2016 Oct 8;9(11):e40303. doi: 10.5812/jjm.40303. eCollection 2016 Nov. Jundishapur J Microbiol. 2016. PMID: 28138375 Free PMC article.
References
-
- Stanley M, Gissmann L, Nardelli-Haefliger D. Immunobiology of human papillomavirus infection and vaccination - implications for second generation vaccines. Vaccine. 2008;26(Suppl 10):K62–7. - PubMed
-
- Lehner T, et al. A rational basis for mucosal vaccination against HIV infection. Immunol Rev. 1999;170:183–96. - PubMed
-
- van Ginkel FW, et al. Cutting edge: the mucosal adjuvant cholera toxin redirects vaccine proteins into olfactory tissues. J Immunol. 2000;165(9):4778–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

