The transcription factor ATF4 promotes skeletal myofiber atrophy during fasting
- PMID: 20197309
- PMCID: PMC2852358
- DOI: 10.1210/me.2009-0345
The transcription factor ATF4 promotes skeletal myofiber atrophy during fasting
Abstract
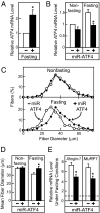
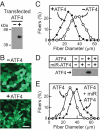
Prolonged fasting alters skeletal muscle gene expression in a manner that promotes myofiber atrophy, but the underlying mechanisms are not fully understood. Here, we examined the potential role of activating transcription factor 4 (ATF4), a transcription factor with an evolutionarily ancient role in the cellular response to starvation. In mouse skeletal muscle, fasting increases the level of ATF4 mRNA. To determine whether increased ATF4 expression was required for myofiber atrophy, we reduced ATF4 expression with an inhibitory RNA targeting ATF4 and found that it reduced myofiber atrophy during fasting. Likewise, reducing the fasting level of ATF4 mRNA with a phosphorylation-resistant form of eukaryotic initiation factor 2alpha decreased myofiber atrophy. To determine whether ATF4 was sufficient to reduce myofiber size, we overexpressed ATF4 and found that it reduced myofiber size in the absence of fasting. In contrast, a transcriptionally inactive ATF4 construct did not reduce myofiber size, suggesting a requirement for ATF4-mediated transcriptional regulation. To begin to determine the mechanism of ATF4-mediated myofiber atrophy, we compared the effects of fasting and ATF4 overexpression on global skeletal muscle mRNA expression. Interestingly, expression of ATF4 increased a small subset of five fasting-responsive mRNAs, including four of the 15 mRNAs most highly induced by fasting. These five mRNAs encode proteins previously implicated in growth suppression (p21(Cip1/Waf1), GADD45alpha, and PW1/Peg3) or titin-based stress signaling [muscle LIM protein (MLP) and cardiac ankyrin repeat protein (CARP)]. Taken together, these data identify ATF4 as a novel mediator of skeletal myofiber atrophy during starvation.
Figures


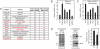
References
-
- Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D 2003 An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11:619–633 - PubMed
-
- Yamaguchi S, Ishihara H, Yamada T, Tamura A, Usui M, Tominaga R, Munakata Y, Satake C, Katagiri H, Tashiro F, Aburatani H, Tsukiyama-Kohara K, Miyazaki J, Sonenberg N, Oka Y 2008 ATF4-mediated induction of 4E-BP1 contributes to pancreatic β-cell survival under endoplasmic reticulum stress. Cell Metab 7:269–276 - PubMed
-
- Wek RC, Cavener DR 2007 Translational control and the unfolded protein response. Antioxid Redox Signal 9:2357–2371 - PubMed
-
- Proud CG 2005 eIF2 and the control of cell physiology. Semin Cell Dev Biol 16:3–12 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

