Involvement of estrogen receptor variant ER-alpha36, not GPR30, in nongenomic estrogen signaling
- PMID: 20197310
- PMCID: PMC2852353
- DOI: 10.1210/me.2009-0317
Involvement of estrogen receptor variant ER-alpha36, not GPR30, in nongenomic estrogen signaling
Abstract
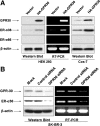
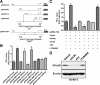
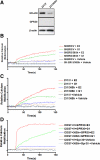
Accumulating evidence suggested that an orphan G protein-coupled receptor (GPR)30, mediates nongenomic responses to estrogen. The present study was performed to investigate the molecular mechanisms underlying GPR30 function. We found that knockdown of GPR30 expression in breast cancer SK-BR-3 cells down-regulated the expression levels of estrogen receptor (ER)-alpha36, a variant of ER-alpha. Introduction of a GPR30 expression vector into GPR30 nonexpressing cells induced endogenous ER-alpha36 expression, and cotransfection assay demonstrated that GPR30 activated the promoter activity of ER-alpha36 via an activator protein 1 binding site. Both 17beta-estradiol (E2) and G1, a compound reported to be a selective GPR30 agonist, increased the phosphorylation levels of the MAPK/ERK1/2 in SK-BR-3 cells, which could be blocked by an anti-ER-alpha36-specific antibody against its ligand-binding domain. G1 induced activities mediated by ER-alpha36, such as transcription activation activity of a VP16-ER-alpha36 fusion protein and activation of the MAPK/ERK1/2 in ER-alpha36-expressing cells. ER-alpha36-expressing cells, but not the nonexpressing cells, displayed high-affinity, specific E2 and G1 binding, and E2- and G1-induced intracellular Ca(2+) mobilization only in ER-alpha36 expressing cells. Taken together, our results demonstrated that previously reported activities of GPR30 in response to estrogen were through its ability to induce ER-alpha36 expression. The selective G protein-coupled receptor (GPR)30 agonist G1 actually interacts with ER-alpha36. Thus, the ER-alpha variant ER-alpha36, not GPR30, is involved in nongenomic estrogen signaling.
Figures



Similar articles
-
GPR30 activation opposes estrogen-dependent uterine growth via inhibition of stromal ERK1/2 and estrogen receptor alpha (ERα) phosphorylation signals.Endocrinology. 2011 Apr;152(4):1434-47. doi: 10.1210/en.2010-1368. Epub 2011 Feb 8. Endocrinology. 2011. PMID: 21303939 Free PMC article.
-
17 beta-estradiol activates rapid signaling pathways involved in rat pachytene spermatocytes apoptosis through GPR30 and ER alpha.Mol Cell Endocrinol. 2010 May 14;320(1-2):136-44. doi: 10.1016/j.mce.2010.01.035. Epub 2010 Feb 2. Mol Cell Endocrinol. 2010. PMID: 20132863
-
Estrogen activation of the mitogen-activated protein kinase is mediated by ER-α36 in ER-positive breast cancer cells.J Steroid Biochem Mol Biol. 2014 Sep;143:434-43. doi: 10.1016/j.jsbmb.2014.06.009. Epub 2014 Jun 25. J Steroid Biochem Mol Biol. 2014. PMID: 24973581
-
Estrogen receptor alpha-36 (ER-α36): A new player in human breast cancer.Mol Cell Endocrinol. 2015 Dec 15;418 Pt 3:193-206. doi: 10.1016/j.mce.2015.04.017. Epub 2015 Apr 24. Mol Cell Endocrinol. 2015. PMID: 25917453 Review.
-
Expression Significance of Estrogen Receptor ER-α36 in Breast Cancer Treated by Chemotherapy: A Meta-Analysis.Mol Biotechnol. 2024 May;66(5):991-999. doi: 10.1007/s12033-023-01029-x. Epub 2024 Jan 25. Mol Biotechnol. 2024. PMID: 38270756 Review.
Cited by
-
An alkylphenol mix promotes seminoma derived cell proliferation through an ERalpha36-mediated mechanism.PLoS One. 2013 Apr 23;8(4):e61758. doi: 10.1371/journal.pone.0061758. Print 2013. PLoS One. 2013. PMID: 23626723 Free PMC article.
-
Minireview: G protein-coupled estrogen receptor-1, GPER-1: its mechanism of action and role in female reproductive cancer, renal and vascular physiology.Endocrinology. 2012 Jul;153(7):2953-62. doi: 10.1210/en.2012-1061. Epub 2012 Apr 11. Endocrinology. 2012. PMID: 22495674 Free PMC article. Review.
-
Mechanism of Rapid Nuclear Factor-E2-Related Factor 2 (Nrf2) Activation via Membrane-Associated Estrogen Receptors: Roles of NADPH Oxidase 1, Neutral Sphingomyelinase 2 and Epidermal Growth Factor Receptor (EGFR).Antioxidants (Basel). 2019 Mar 18;8(3):69. doi: 10.3390/antiox8030069. Antioxidants (Basel). 2019. PMID: 30889865 Free PMC article. Review.
-
Missing Information from the Estrogen Receptor Puzzle: Where Are They Localized in Bull Reproductive Tissues and Spermatozoa?Cells. 2020 Jan 10;9(1):183. doi: 10.3390/cells9010183. Cells. 2020. PMID: 31936899 Free PMC article.
-
G protein-coupled oestrogen receptor 1 (GPER1)/GPR30: a new player in cardiovascular and metabolic oestrogenic signalling.Br J Pharmacol. 2011 Jul;163(6):1131-9. doi: 10.1111/j.1476-5381.2011.01235.x. Br J Pharmacol. 2011. PMID: 21250980 Free PMC article. Review.
References
-
- Tsai MJ, O'Malley BW 1994 Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem 63:451–486 - PubMed
-
- Hammes SR, Levin ER 2007 Extranuclear steroid receptors: nature and actions. Endocr Rev 28:726–741 - PubMed
-
- McDonnell DP, Norris JD 2002 Connections and regulation of the human estrogen receptor. Science 296:1642–1644 - PubMed
-
- Hall JM, Couse JF, Korach KS 2001 The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem 276: 36869–36872 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

