Risk of miscarriage with bivalent vaccine against human papillomavirus (HPV) types 16 and 18: pooled analysis of two randomised controlled trials
- PMID: 20197322
- PMCID: PMC2831171
- DOI: 10.1136/bmj.c712
Risk of miscarriage with bivalent vaccine against human papillomavirus (HPV) types 16 and 18: pooled analysis of two randomised controlled trials
Abstract
Objective: To assess whether vaccination against human papillomavirus (HPV) increases the risk of miscarriage.
Design: Pooled analysis of two multicentre, phase three masked randomised controlled trials
Setting: Multicentre trials in several continents and in Costa Rica.
Participants: 26 130 women aged 15-25 at enrolment; 3599 pregnancies eligible for analysis.
Interventions: Participants were randomly assigned to receive three doses of bivalent HPV 16/18 VLP vaccine with AS04 adjuvant (n=13 075) or hepatitis A vaccine as control (n=13 055) over six months.
Main outcome measures: Miscarriage and other pregnancy outcomes.
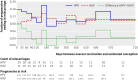
Results: The estimated rate of miscarriage was 11.5% in pregnancies in women in the HPV arm and 10.2% in the control arm. The one sided P value for the primary analysis was 0.16; thus, overall, there was no significant increase in miscarriage among women assigned to the HPV vaccine arm. In secondary descriptive analyses, miscarriage rates were 14.7% in the HPV vaccine arm and 9.1% in the control arm in pregnancies that began within three months after nearest vaccination.
Conclusion: There is no evidence overall for an association between HPV vaccination and risk of miscarriage.
Trial registration: Clinical Trials NCT00128661 and NCT00122681.
Conflict of interest statement
Competing interests: Vaccine was provided for CVT by GSK Biologicals, under a clinical trials agreement with NCI. GSK also provided support for aspects of the trial associated with regulatory submission needs of the company under FDA BB-IND 7920. Douglas Lowy and John Schiller from NCI are named inventors on US government owned HPV vaccine patents that are licensed to GSK and Merck, and so are entitled to limited royalties as specified by federal law. None of the other NCI and Costa Rica co-authors have any potential conflicts of interest to report. The researchers are completely independent from the non-government funders and sponsors. After a planned interim analysis, including safety review, of PATRICIA in December 2006, the data and safety monitoring board (DSMB) of CVT requested an assessment of possible effects of the vaccine on miscarriages by performing a pooled post hoc analysis of data from the two parallel trials. GSK provided data on pregnancy for this miscarriage analysis from PATRICIA to the statistician for CVT (SW). Two outside consultants (GM and AW), who are experts in reproductive epidemiology, helped in the preparation of the analysis and report. Both the data and safety monitoring board and the independent data monitoring committee (IDMC), which oversees CVT and PATRICIA trials, respectively, recommended that NCI statistician prepare a manuscript describing the results for publication in the scientific literature. GSK scientists provided background information and data from PATRICIA, and provided suggestions on the methods, analysis and interpretation. CVT investigators from NCI and Costa Rica prepared this manuscript with input from the expert and consultants. GSK scientists commented on draft manuscripts, but the named authors made the final decisions about its content.
Figures
Comment in
-
Monitoring HPV vaccination programmes.BMJ. 2010 Mar 24;340:c1666. doi: 10.1136/bmj.c1666. BMJ. 2010. PMID: 20335330 No abstract available.
References
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74-108. - PubMed
-
- Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet 2007;370:890-907. - PubMed
-
- Paavonen J, Jenkins D, Bosch FX, Naud P, Salmeron J, Wheeler CM, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet 2007;369:2161-70. - PubMed
-
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007;356:1915-27. - PubMed
-
- Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 2007;356:1928-43. - PubMed
