Changes in Bni4 localization induced by cell stress in Saccharomyces cerevisiae
- PMID: 20197406
- PMCID: PMC2844317
- DOI: 10.1242/jcs.066258
Changes in Bni4 localization induced by cell stress in Saccharomyces cerevisiae
Abstract
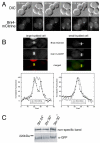
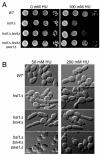
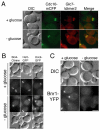
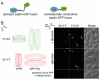
Septin complexes at the bud neck in Saccharomyces cerevisiae serve as a scaffold for proteins involved in signaling, cell cycle control, and cell wall synthesis. Many of these bind asymmetrically, associating with either the mother- or daughter-side of the neck. Septin structures are inherently apolar so the basis for the asymmetric binding remains unknown. Bni4, a regulatory subunit of yeast protein phosphatase type 1, Glc7, binds to the outside of the septin ring prior to bud formation and remains restricted to the mother-side of the bud neck after bud emergence. Bni4 is responsible for targeting Glc7 to the mother-side of the bud neck for proper deposition of the chitin ring. We show here that Bni4 localizes symmetrically, as two distinct rings on both sides of the bud neck following energy depletion or activation of cell cycle checkpoints. Our data indicate that loss of Bni4 asymmetry can occur via at least two different mechanisms. Furthermore, we show that Bni4 has a Swe1-dependent role in regulating the cell morphogenesis checkpoint in response to hydroxyurea, which suggests that the change in localization of Bni4 following checkpoint activation may help stabilize the cell cycle regulator Swe1 during cell cycle arrest.
Figures



References
-
- Bertin A., McMurray M. A., Grob P., Park S. S., Garcia G., 3rd, Patanwala I., Ng H. L., Alber T., Thorner J., Nogales E. (2008). Saccharomyces cerevisiae septins: supramolecular organization of heterooligomers and the mechanism of filament assembly. Proc. Natl. Acad. Sci. USA 105, 8274-8279 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

