Ascidians: an invertebrate chordate model to study Alzheimer's disease pathogenesis
- PMID: 20197417
- PMCID: PMC4068634
- DOI: 10.1242/dmm.003434
Ascidians: an invertebrate chordate model to study Alzheimer's disease pathogenesis
Abstract
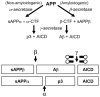
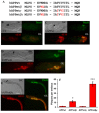
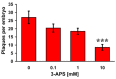
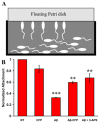
Here we present the ascidian Ciona intestinalis as an alternative invertebrate system to study Alzheimer's disease (AD) pathogenesis. Through the use of AD animal models, researchers often attempt to reproduce various aspects of the disease, particularly the coordinated processing of the amyloid precursor protein (APP) by alpha-, beta- and gamma-secretases to generate amyloid beta (Abeta)-containing plaques. Recently, Drosophila and C. elegans AD models have been developed, exploiting the relative simplicity of these invertebrate systems, but they lack a functional Abeta sequence and a beta-secretase ortholog, thus complicating efforts to examine APP processing in vivo. We propose that the ascidian is a more appropriate invertebrate AD model owing to their phylogenetic relationship with humans. This is supported by bioinformatic analyses, which indicate that the ascidian genome contains orthologs of all AD-relevant genes. We report that transgenic ascidian larvae can properly process human APP(695) to generate Abeta peptides. Furthermore, Abeta can rapidly aggregate to form amyloid-like plaques, and plaque deposition is significantly increased in larvae expressing a human APP(695) variant associated with familial Alzheimer's disease. We also demonstrate that nervous system-specific Abeta expression alters normal larval behavior during attachment. Importantly, plaque formation and alterations in behavior are not only observed within 24 hours post-fertilization, but anti-amyloid drug treatment improves these AD-like pathologies. This ascidian model for AD provides a powerful and rapid system to study APP processing, Abeta plaque formation and behavioral alterations, and could aid in identifying factors that modulate amyloid deposition and the associated disruption of normal cellular function and behaviors.
Figures


Similar articles
-
[Alzheimer disease: cellular and molecular aspects].Bull Mem Acad R Med Belg. 2005;160(10-12):445-9; discussion 450-1. Bull Mem Acad R Med Belg. 2005. PMID: 16768248 French.
-
Proteolytic processing of Alzheimer's β-amyloid precursor protein.J Neurochem. 2012 Jan;120 Suppl 1(Suppl 1):9-21. doi: 10.1111/j.1471-4159.2011.07519.x. Epub 2011 Nov 28. J Neurochem. 2012. PMID: 22122372 Free PMC article. Review.
-
The role of amyloid in the pathogenesis of Alzheimer's disease.Biol Chem. 1997 Sep;378(9):937-50. doi: 10.1515/bchm.1997.378.9.937. Biol Chem. 1997. PMID: 9348103 Review.
-
Amyloid-β protein (Aβ) Glu11 is the major β-secretase site of β-site amyloid-β precursor protein-cleaving enzyme 1(BACE1), and shifting the cleavage site to Aβ Asp1 contributes to Alzheimer pathogenesis.Eur J Neurosci. 2013 Jun;37(12):1962-9. doi: 10.1111/ejn.12235. Eur J Neurosci. 2013. PMID: 23773065
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
Cited by
-
The Simple Chordate Ciona intestinalis Has a Reduced Complement of Genes Associated with Fanconi Anemia.Evol Bioinform Online. 2016 Jun 6;12:133-48. doi: 10.4137/EBO.S37920. eCollection 2016. Evol Bioinform Online. 2016. PMID: 27279728 Free PMC article.
-
Multiple Forms of Neural Cell Death in the Cyclical Brain Degeneration of A Colonial Chordate.Cells. 2023 Mar 29;12(7):1041. doi: 10.3390/cells12071041. Cells. 2023. PMID: 37048113 Free PMC article.
-
Tunicates: exploring the sea shores and roaming the open ocean. A tribute to Thomas Huxley.Open Biol. 2015 Jun;5(6):150053. doi: 10.1098/rsob.150053. Open Biol. 2015. PMID: 26085517 Free PMC article. Review.
-
Neuronal migration during development and the amyloid precursor protein.Curr Opin Insect Sci. 2016 Dec;18:1-10. doi: 10.1016/j.cois.2016.08.001. Epub 2016 Aug 16. Curr Opin Insect Sci. 2016. PMID: 27939704 Free PMC article. Review.
-
Fine-Tuned Expression of Evolutionarily Conserved Signaling Molecules in the Ciona Notochord.Int J Mol Sci. 2024 Dec 20;25(24):13631. doi: 10.3390/ijms252413631. Int J Mol Sci. 2024. PMID: 39769393 Free PMC article.
References
-
- Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. (2007). Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement. 3, 186–191 - PubMed
-
- Buckig A, Tikkanen R, Herzog V, Schmitz A. (2002). Cytosolic and nuclear aggregation of the amyloid beta-peptide following its expression in the endoplasmic reticulum. Histochem Cell Biol. 118, 353–360 - PubMed
-
- Chambon JP, Soule J, Pomies P, Fort P, Sahuquet A, Alexandre D, Mangeat PH, Baghdiguian S. (2002). Tail regression in Ciona intestinalis (prochordate) involves a caspase-dependent apoptosis event associated with ERK activation. Development 129, 3105–3114 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

