Step-up therapy for children with uncontrolled asthma receiving inhaled corticosteroids
- PMID: 20197425
- PMCID: PMC2989902
- DOI: 10.1056/NEJMoa1001278
Step-up therapy for children with uncontrolled asthma receiving inhaled corticosteroids
Abstract
Background: For children who have uncontrolled asthma despite the use of low-dose inhaled corticosteroids (ICS), evidence to guide step-up therapy is lacking.
Methods: We randomly assigned 182 children (6 to 17 years of age), who had uncontrolled asthma while receiving 100 microg of fluticasone twice daily, to receive each of three blinded step-up therapies in random order for 16 weeks: 250 microg of fluticasone twice daily (ICS step-up), 100 microg of fluticasone plus 50 microg of a long-acting beta-agonist twice daily (LABA step-up), or 100 microg of fluticasone twice daily plus 5 or 10 mg of a leukotriene-receptor antagonist daily (LTRA step-up). We used a triple-crossover design and a composite of three outcomes (exacerbations, asthma-control days, and the forced expiratory volume in 1 second) to determine whether the frequency of a differential response to the step-up regimens was more than 25%.
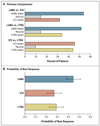
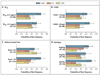
Results: A differential response occurred in 161 of 165 patients who were evaluated (P<0.001). The response to LABA step-up therapy was most likely to be the best response, as compared with responses to LTRA step-up (relative probability, 1.6; 95% confidence interval [CI], 1.1 to 2.3; P=0.004) and ICS step-up (relative probability, 1.7; 95% CI, 1.2 to 2.4; P=0.002). Higher scores on the Asthma Control Test before randomization (indicating better control at baseline) predicted a better response to LABA step-up (P=0.009). White race predicted a better response to LABA step-up, whereas black patients were least likely to have a best response to LTRA step-up (P=0.005).
Conclusions: Nearly all the children had a differential response to each step-up therapy. LABA step-up was significantly more likely to provide the best response than either ICS or LTRA step-up. However, many children had a best response to ICS or LTRA step-up therapy, highlighting the need to regularly monitor and appropriately adjust each child's asthma therapy. (ClinicalTrials.gov number, NCT00395304.)
2010 Massachusetts Medical Society
Conflict of interest statement
No other potential conflict of interest relevant to this article was reported.
Figures


Comment in
-
Choosing asthma step-up care.N Engl J Med. 2010 Mar 18;362(11):1042-3. doi: 10.1056/NEJMe1002058. Epub 2010 Mar 2. N Engl J Med. 2010. PMID: 20197426 No abstract available.
-
Step-up therapy for children with uncontrolled asthma.N Engl J Med. 2010 Jul 1;363(1):90; author reply 91-2. doi: 10.1056/NEJMc1004307. N Engl J Med. 2010. PMID: 20592302 No abstract available.
-
Step-up therapy for children with uncontrolled asthma.N Engl J Med. 2010 Jul 1;363(1):90-1; author reply 91-2. N Engl J Med. 2010. PMID: 20597147 No abstract available.
-
Step-up therapy for children with uncontrolled asthma.N Engl J Med. 2010 Jul 1;363(1):91; author reply 91-2. N Engl J Med. 2010. PMID: 20597148 No abstract available.
-
Long-acting ß-agonist step-up therapy is more likely to provide best response, compared to inhaled corticosteroid or leukotriene-receptor antagonist step-up in children with uncontrolled asthma receiving inhaled corticosteroids.Evid Based Med. 2010 Dec;15(6):167-8. doi: 10.1136/ebm1117. Epub 2010 Aug 16. Evid Based Med. 2010. PMID: 20713541 No abstract available.
-
Long-acting beta--agonists best option for "step-up" therapy for children with uncontrolled asthma.J Pediatr. 2010 Sep;157(3):512-3. doi: 10.1016/j.jpeds.2010.07.015. J Pediatr. 2010. PMID: 20727443 No abstract available.
References
-
- Sorkness CA, Lemanske RF, Jr, Mauger DT, et al. Long-term comparison of 3 controller regimens for mild-moderate persistent childhood asthma: the Pediatric Asthma Controller Trial. J Allergy Clin Immunol. 2007;119:64–72. - PubMed
-
- Verberne AA, Frost C, Duiverman EJ, Grol MH, Kerrebijn KF. Addition of salmeterol versus doubling the dose of beclomethasone in children with asthma. Am J Respir Crit Care Med. 1998;158:213–219. - PubMed
-
- Bensch G, Berger WE, Blokhin BM, et al. One-year efficacy and safety of inhaled formoterol dry powder in children with persistent asthma. Ann Allergy Asthma Immunol. 2002;89:180–190. - PubMed
-
- de Blic J, Ogorodova L, Klink R, et al. Salmeterol/fluticasone propionate vs. double dose fluticasone propionate on lung function and asthma control in children. Pediatr Allergy Immunol. 2009;20:763–771. - PubMed
-
- Gappa M, Zachgo W, von Berg A, Kamin W, Stern-Sträter C, Steinkamp G. Add-on salmeterol compared to double dose fluticasone in pediatric asthma: a double-blind randomized trial (VIAPAED) Pediatr Pulmonol. 2009;44:1132–1142. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- M01-RR03186/RR/NCRR NIH HHS/United States
- HL064288/HL/NHLBI NIH HHS/United States
- UL1 RR025780/RR/NCRR NIH HHS/United States
- T32AI007635/AI/NIAID NIH HHS/United States
- U10 HL064288/HL/NHLBI NIH HHS/United States
- U10 HL064287/HL/NHLBI NIH HHS/United States
- UL1 RR025011/RR/NCRR NIH HHS/United States
- U10 HL064305/HL/NHLBI NIH HHS/United States
- HL064307/HL/NHLBI NIH HHS/United States
- HL064295/HL/NHLBI NIH HHS/United States
- U10 HL064313/HL/NHLBI NIH HHS/United States
- UL1-RR025011/RR/NCRR NIH HHS/United States
- M01 RR003186/RR/NCRR NIH HHS/United States
- U10 HL064307/HL/NHLBI NIH HHS/United States
- M01 RR000036/RR/NCRR NIH HHS/United States
- T32 AI007635/AI/NIAID NIH HHS/United States
- HL064287/HL/NHLBI NIH HHS/United States
- U10 HL064295/HL/NHLBI NIH HHS/United States
- UL1 RR024992/RR/NCRR NIH HHS/United States
- HL064305/HL/NHLBI NIH HHS/United States
- HL064313/HL/NHLBI NIH HHS/United States
- M01 RR000051/RR/NCRR NIH HHS/United States
- M01-RR00051/RR/NCRR NIH HHS/United States
- M01-RR00036/RR/NCRR NIH HHS/United States
- UL1-RR025780/RR/NCRR NIH HHS/United States
- UL1-RR024992/RR/NCRR NIH HHS/United States
- UL1 TR000448/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
