Simultaneous visualization of protumorigenic Src and MT1-MMP activities with fluorescence resonance energy transfer
- PMID: 20197470
- PMCID: PMC2840183
- DOI: 10.1158/0008-5472.CAN-09-3698
Simultaneous visualization of protumorigenic Src and MT1-MMP activities with fluorescence resonance energy transfer
Abstract
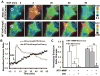
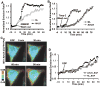
Both Src kinase and membrane type 1 matrix metalloproteinase (MT1-MMP) play critical roles in cancer invasion and metastasis. It is not clear, however, how the spatiotemporal activation of these two critical enzymes is coordinated in response to an oncogenic epithelial growth factor (EGF) stimulation. Here, we have visualized the activities of Src and MT1-MMP concurrently in a single live cell by combining two fluorescence resonance energy transfer (FRET) pairs with distinct spectra: (a) cyan fluorescent protein (CFP) and yellow FP (YFP), and (b) orange FP (mOrange2) and red FP (mCherry). The new FRET pair, mOrange2 and mCherry, was first characterized in vitro and in cultured mammalian cells. When integrated with the CFP/YFP pair, this new pair allowed the revelation of an immediate, rapid, and relatively dispersed Src activity. In contrast, the MT1-MMP activity displayed a slow increase at the cell periphery, although Src was shown to play a role upstream to MT1-MMP globally. This difference in the activation patterns of MT1-MMP and Src in response to EGF is further confirmed using an optimized MT1-MMP biosensor capable of being rapidly cleaved by MT1-MMP. The results indicate that although Src and MT1-MMP act globally in the same signaling pathway, their activations differ in space and time upon EGF stimulation, possibly mediated by different sets of intermediates at different subcellular locations. Our results also showed the potential of mOrange2/mCherry as a new FRET pair, together with the popular variants of CFP and YFP, for the simultaneous visualization of multiple molecular activities in a single live cell.
Figures



Similar articles
-
Visualization of polarized membrane type 1 matrix metalloproteinase activity in live cells by fluorescence resonance energy transfer imaging.J Biol Chem. 2008 Jun 20;283(25):17740-8. doi: 10.1074/jbc.M709872200. Epub 2008 Apr 25. J Biol Chem. 2008. PMID: 18441011 Free PMC article.
-
Determination of hierarchical relationship of Src and Rac at subcellular locations with FRET biosensors.Proc Natl Acad Sci U S A. 2008 Sep 23;105(38):14353-8. doi: 10.1073/pnas.0807537105. Epub 2008 Sep 17. Proc Natl Acad Sci U S A. 2008. PMID: 18799748 Free PMC article.
-
Activatable and Cell-Penetrable Multiplex FRET Nanosensor for Profiling MT1-MMP Activity in Single Cancer Cells.Nano Lett. 2015 Aug 12;15(8):5025-32. doi: 10.1021/acs.nanolett.5b01047. Epub 2015 Jul 27. Nano Lett. 2015. PMID: 26203778 Free PMC article.
-
Genetically encoded fluorescent biosensors for live-cell imaging of MT1-MMP protease activity.Methods Mol Biol. 2014;1071:163-74. doi: 10.1007/978-1-62703-622-1_13. Methods Mol Biol. 2014. PMID: 24052388 Free PMC article.
-
Novel Roles of MT1-MMP and MMP-2: Beyond the Extracellular Milieu.Int J Mol Sci. 2022 Aug 23;23(17):9513. doi: 10.3390/ijms23179513. Int J Mol Sci. 2022. PMID: 36076910 Free PMC article. Review.
Cited by
-
Intersection of stem cell biology and engineering towards next generation in vitro models of human fibrosis.Front Bioeng Biotechnol. 2022 Oct 20;10:1005051. doi: 10.3389/fbioe.2022.1005051. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36338120 Free PMC article. Review.
-
Live imaging of apoptotic signaling flow using tunable combinatorial FRET-based bioprobes for cell population analysis of caspase cascades.Sci Rep. 2022 Dec 7;12(1):21160. doi: 10.1038/s41598-022-25286-z. Sci Rep. 2022. PMID: 36476686 Free PMC article.
-
A biosensor for the activity of the "sheddase" TACE (ADAM17) reveals novel and cell type-specific mechanisms of TACE activation.Sci Signal. 2015 Feb 24;8(365):rs1. doi: 10.1126/scisignal.2005680. Sci Signal. 2015. PMID: 25714465 Free PMC article.
-
Label-Free Assay of Protein Tyrosine Phosphatase Activity in Single Cells.Anal Chem. 2019 Oct 15;91(20):13206-13212. doi: 10.1021/acs.analchem.9b03640. Epub 2019 Sep 26. Anal Chem. 2019. PMID: 31536703 Free PMC article.
-
Directed Evolution to Engineer Monobody for FRET Biosensor Assembly and Imaging at Live-Cell Surface.Cell Chem Biol. 2018 Apr 19;25(4):370-379.e4. doi: 10.1016/j.chembiol.2018.01.002. Epub 2018 Jan 27. Cell Chem Biol. 2018. PMID: 29396288 Free PMC article.
References
-
- Seiki M. Membrane-type 1 matrix metalloproteinase: a key enzyme for tumor invasion. Cancer Lett. 2003;194:1–11. - PubMed
-
- Osenkowski P, Toth M, Fridman R. Processing, shedding, and endocytosis of membrane type 1-matrix metalloproteinase (MT1-MMP) J Cell Physiol. 2004;200:2–10. - PubMed
-
- Itoh Y, Seiki M. MT1-MMP: a potent modifier of pericellular microenvironment. J Cell Physiol. 2006;206:1–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

