Getting in the loop: regulation of development in Caulobacter crescentus
- PMID: 20197497
- PMCID: PMC2832345
- DOI: 10.1128/MMBR.00040-09
Getting in the loop: regulation of development in Caulobacter crescentus
Abstract
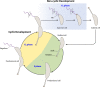
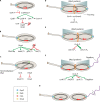
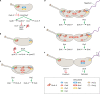
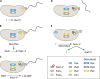
Caulobacter crescentus is an aquatic Gram-negative alphaproteobacterium that undergoes multiple changes in cell shape, organelle production, subcellular distribution of proteins, and intracellular signaling throughout its life cycle. Over 40 years of research has been dedicated to this organism and its developmental life cycles. Here we review a portion of many developmental processes, with particular emphasis on how multiple processes are integrated and coordinated both spatially and temporally. While much has been discovered about Caulobacter crescentus development, areas of potential future research are also highlighted.
Figures
References
-
- Aaron, M., G. Charbon, H. Lam, H. Schwarz, W. Vollmer, and C. Jacobs-Wagner. 2007. The tubulin homologue FtsZ contributes to cell elongation by guiding cell wall precursor synthesis in Caulobacter crescentus. Mol. Microbiol. 64:938-952. - PubMed
-
- Aldridge, P., and K. T. Hughes. 2002. Regulation of flagellar assembly. Curr. Opin. Microbiol. 5:160-165. - PubMed
-
- Aldridge, P., and U. Jenal. 1999. Cell cycle-dependent degradation of a flagellar motor component requires a novel-type response regulator. Mol. Microbiol. 32:379-391. - PubMed
-
- Aldridge, P., R. Paul, P. Goymer, P. Rainey, and U. Jenal. 2003. Role of the GGDEF regulator PleD in polar development of Caulobacter crescentus. Mol. Microbiol. 47:1695-1708. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

