Virulence and immunomodulatory roles of bacterial outer membrane vesicles
- PMID: 20197500
- PMCID: PMC2832350
- DOI: 10.1128/MMBR.00031-09
Virulence and immunomodulatory roles of bacterial outer membrane vesicles
Abstract
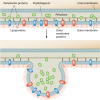
Outer membrane (OM) vesicles are ubiquitously produced by Gram-negative bacteria during all stages of bacterial growth. OM vesicles are naturally secreted by both pathogenic and nonpathogenic bacteria. Strong experimental evidence exists to categorize OM vesicle production as a type of Gram-negative bacterial virulence factor. A growing body of data demonstrates an association of active virulence factors and toxins with vesicles, suggesting that they play a role in pathogenesis. One of the most popular and best-studied pathogenic functions for membrane vesicles is to serve as natural vehicles for the intercellular transport of virulence factors and other materials directly into host cells. The production of OM vesicles has been identified as an independent bacterial stress response pathway that is activated when bacteria encounter environmental stress, such as what might be experienced during the colonization of host tissues. Their detection in infected human tissues reinforces this theory. Various other virulence factors are also associated with OM vesicles, including adhesins and degradative enzymes. As a result, OM vesicles are heavily laden with pathogen-associated molecular patterns (PAMPs), virulence factors, and other OM components that can impact the course of infection by having toxigenic effects or by the activation of the innate immune response. However, infected hosts can also benefit from OM vesicle production by stimulating their ability to mount an effective defense. Vesicles display antigens and can elicit potent inflammatory and immune responses. In sum, OM vesicles are likely to play a significant role in the virulence of Gram-negative bacterial pathogens.
Figures


References
-
- Alaniz, R. C., B. L. Deatherage, J. C. Lara, and B. T. Cookson. 2007. Membrane vesicles are immunogenic facsimiles of Salmonella typhimurium that potently activate dendritic cells, prime B and T cell responses, and stimulate protective immunity in vivo. J. Immunol. 179:7692-7701. - PubMed
-
- Allan, N. D., C. Kooi, P. A. Sokol, and T. J. Beveridge. 2003. Putative virulence factors are released in association with membrane vesicles from Burkholderia cepacia. Can. J. Microbiol. 49:613-624. - PubMed
-
- Aoki, M., M. Kondo, Y. Nakatsuka, K. Kawai, and S. Oshima. 2007. Stationary phase culture supernatant containing membrane vesicles induced immunity to rainbow trout Oncorhynchus mykiss fry syndrome. Vaccine 25:561-569. - PubMed
-
- Ayala, G., L. Torres, M. Espinosa, G. Fierros-Zarate, V. Maldonado, and J. Melendez-Zajgla. 2006. External membrane vesicles from Helicobacter pylori induce apoptosis in gastric epithelial cells. FEMS Microbiol. Lett. 260:178-185. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

