Alkali metal cation transport and homeostasis in yeasts
- PMID: 20197501
- PMCID: PMC2832347
- DOI: 10.1128/MMBR.00042-09
Alkali metal cation transport and homeostasis in yeasts
Abstract
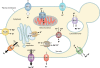
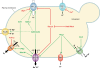
The maintenance of appropriate intracellular concentrations of alkali metal cations, principally K(+) and Na(+), is of utmost importance for living cells, since they determine cell volume, intracellular pH, and potential across the plasma membrane, among other important cellular parameters. Yeasts have developed a number of strategies to adapt to large variations in the concentrations of these cations in the environment, basically by controlling transport processes. Plasma membrane high-affinity K(+) transporters allow intracellular accumulation of this cation even when it is scarce in the environment. Exposure to high concentrations of Na(+) can be tolerated due to the existence of an Na(+), K(+)-ATPase and an Na(+), K(+)/H(+)-antiporter, which contribute to the potassium balance as well. Cations can also be sequestered through various antiporters into intracellular organelles, such as the vacuole. Although some uncertainties still persist, the nature of the major structural components responsible for alkali metal cation fluxes across yeast membranes has been defined within the last 20 years. In contrast, the regulatory components and their interactions are, in many cases, still unclear. Conserved signaling pathways (e.g., calcineurin and HOG) are known to participate in the regulation of influx and efflux processes at the plasma membrane level, even though the molecular details are obscure. Similarly, very little is known about the regulation of organellar transport and homeostasis of alkali metal cations. The aim of this review is to provide a comprehensive and up-to-date vision of the mechanisms responsible for alkali metal cation transport and their regulation in the model yeast Saccharomyces cerevisiae and to establish, when possible, comparisons with other yeasts and higher plants.
Figures


References
-
- Ahmed, A., F. Sesti, N. Ilan, T. M. Shih, S. L. Sturley, and S. A. Goldstein. 1999. A molecular target for viral killer toxin: TOK1 potassium channels. Cell 99:283-291. - PubMed
-
- Alepuz, P. M., K. W. Cunningham, and F. Estruch. 1997. Glucose repression affects ion homeostasis in yeast through the regulation of the stress-activated ENA1 gene. Mol. Microbiol. 26:91-98. - PubMed
-
- Reference deleted.
-
- Ali, R., C. L. Brett, S. Mukherjee, and R. Rao. 2004. Inhibition of sodium/proton exchange by a Rab-GTPase-activating protein regulates endosomal traffic in yeast. J. Biol. Chem. 279:4498-4506. - PubMed
-
- Alijo, R., and J. Ramos. 1993. Several routes of activation of the potassium uptake system of yeast. Biochim. Biophys. Acta 1179:224-228. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

