Internal architecture of mitochondrial complex I from Arabidopsis thaliana
- PMID: 20197505
- PMCID: PMC2861459
- DOI: 10.1105/tpc.109.073726
Internal architecture of mitochondrial complex I from Arabidopsis thaliana
Abstract
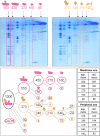
The NADH dehydrogenase complex (complex I) of the respiratory chain has unique features in plants. It is the main entrance site for electrons into the respiratory electron transfer chain, has a role in maintaining the redox balance of the entire plant cell and additionally comprises enzymatic side activities essential for other metabolic pathways. Here, we present a proteomic investigation to elucidate its internal structure. Arabidopsis thaliana complex I was purified by a gentle biochemical procedure that includes a cytochrome c-mediated depletion of other respiratory protein complexes. To examine its internal subunit arrangement, isolated complex I was dissected into subcomplexes. Controlled disassembly of the holo complex (1000 kD) by low-concentration SDS treatment produced 10 subcomplexes of 550, 450, 370, 270, 240, 210, 160, 140, 140, and 85 kD. Systematic analyses of subunit composition by mass spectrometry gave insights into subunit arrangement within complex I. Overall, Arabidopsis complex I includes at least 49 subunits, 17 of which are unique to plants. Subunits form subcomplexes analogous to the known functional modules of complex I from heterotrophic eukaryotes (the so-called N-, Q-, and P-modules), but also additional modules, most notably an 85-kD domain including gamma-type carbonic anhydrases. Based on topological information for many of its subunits, we present a model of the internal architecture of plant complex I.
Figures








References
-
- Abdrakhmanova A., Zickermann V., Bostina M., Radermacher M., Schägger H., Kerscher S., Brandt U. (2004). Subunit composition of mitochondrial complex I from the yeast Yarrowia lipolytica. Biochim. Biophys. Acta 1658: 148–156 - PubMed
-
- Allen K.D., Staehelin L.A. (1991). Resolution of 16 to 20 chlorophyll-protein complexes using a low ionic strength native green gel system. Anal. Biochem. 194: 214–222 - PubMed
-
- Arizmendi J.M., Skehel J.M., Runswick M.J., Fearnley I.M., Walker J.E. (1992). Complementary DNA sequences of two 14.5 kDa subunits of NADH:ubiquinone oxidoreductase from bovine heart mitochondria. Completion of the primary structure of the complex? FEBS Lett. 313: 80–84 - PubMed
-
- Baranova E.A., Holt P.J., Sazanov L.A. (2007b). Projection structure of the membrane domain of Escherichia coli respiratory complex I at 8 A resolution. J. Mol. Biol. 366: 140–154 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

