Crystal structure of the alpha-kinase domain of Dictyostelium myosin heavy chain kinase A
- PMID: 20197546
- PMCID: PMC2894936
- DOI: 10.1126/scisignal.2000525
Crystal structure of the alpha-kinase domain of Dictyostelium myosin heavy chain kinase A
Abstract
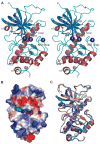
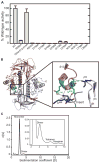
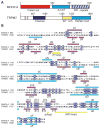
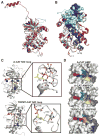
Dictyostelium discoideum myosin II heavy chain kinase A (MHCK A) disrupts the assembly and cellular activity of bipolar filaments of myosin II by phosphorylating sites within its alpha-helical, coiled-coil tail. MHCK A is a member of the atypical alpha-kinase family of serine and threonine protein kinases and displays no sequence homology to typical eukaryotic protein kinases. We report the crystal structure of the alpha-kinase domain (A-CAT) of MHCK A. When crystallized in the presence of adenosine triphosphate (ATP), A-CAT contained adenosine monophosphate (AMP) at the active site. However, when crystallized in the presence of ATP and a peptide substrate, which does not appear in the structure, adenosine diphosphate (ADP) was found at the active site and an invariant aspartic acid residue (Asp(766)) at the active site was phosphorylated. The aspartylphosphate group was exposed to the solvent within an active-site pocket that might function as a docking site for substrates. Access to the aspartylphosphate was regulated by a conformational switch in a loop that bound to a magnesium ion (Mg(2+)), providing a mechanism that allows alpha-kinases to sense and respond to local changes in Mg(2+).
Conflict of interest statement
Competing interests: The authors declare no conflicts of interest.
Figures
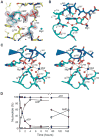
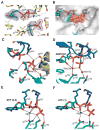
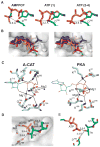
References
-
- De la Roche MA, Smith JL, Betapudi V, Egelhoff TT, Côté GP. Signaling pathways regulating Dictyostelium myosin II. J Muscle Res Cell Motil. 2002;23:703–718. - PubMed
-
- Bosgraaf L, van Haastert PJ. The regulation of myosin II in Dictyostelium. Eur J Cell Biol. 2006;85:969–979. - PubMed
-
- Côté GP, Bukiejko U. Purification and characterization of a myosin heavy chain kinase from Dictyostelium discoideum. J Biol Chem. 1987;262:1065–1072. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

