A novel type of cellular senescence that can be enhanced in mouse models and human tumor xenografts to suppress prostate tumorigenesis
- PMID: 20197621
- PMCID: PMC2827955
- DOI: 10.1172/JCI40535
A novel type of cellular senescence that can be enhanced in mouse models and human tumor xenografts to suppress prostate tumorigenesis
Abstract
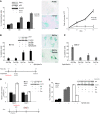
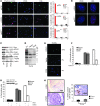
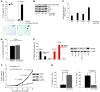
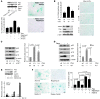
Irreversible cell growth arrest, a process termed cellular senescence, is emerging as an intrinsic tumor suppressive mechanism. Oncogene-induced senescence is thought to be invariably preceded by hyperproliferation, aberrant replication, and activation of a DNA damage checkpoint response (DDR), rendering therapeutic enhancement of this process unsuitable for cancer treatment. We previously demonstrated in a mouse model of prostate cancer that inactivation of the tumor suppressor phosphatase and tensin homolog deleted on chromosome 10 (Pten) elicits a senescence response that opposes tumorigenesis. Here, we show that Pten-loss-induced cellular senescence (PICS) represents a senescence response that is distinct from oncogene-induced senescence and can be targeted for cancer therapy. Using mouse embryonic fibroblasts, we determined that PICS occurs rapidly after Pten inactivation, in the absence of cellular proliferation and DDR. Further, we found that PICS is associated with enhanced p53 translation. Consistent with these data, we showed that in mice p53-stabilizing drugs potentiated PICS and its tumor suppressive potential. Importantly, we demonstrated that pharmacological inhibition of PTEN drives senescence and inhibits tumorigenesis in vivo in a human xenograft model of prostate cancer. Taken together, our data identify a type of cellular senescence that can be triggered in nonproliferating cells in the absence of DNA damage, which we believe will be useful for developing a "pro-senescence" approach for cancer prevention and therapy.
Figures
Comment in
-
Exploring a 'pro-senescence' approach for prostate cancer therapy by targeting PTEN.Future Oncol. 2010 May;6(5):687-9. doi: 10.2217/fon.10.39. Future Oncol. 2010. PMID: 20465384 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

