The von Hippel-Lindau Chuvash mutation promotes pulmonary hypertension and fibrosis in mice
- PMID: 20197624
- PMCID: PMC2827942
- DOI: 10.1172/JCI36362
The von Hippel-Lindau Chuvash mutation promotes pulmonary hypertension and fibrosis in mice
Abstract
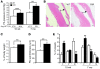
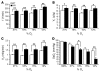
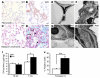
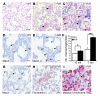
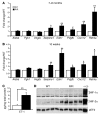
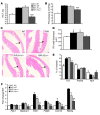
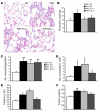
Mutation of the von Hippel-Lindau (VHL) tumor suppressor protein at codon 200 (R200W) is associated with a disease known as Chuvash polycythemia. In addition to polycythemia, Chuvash patients have pulmonary hypertension and increased respiratory rates, although the pathophysiological basis of these symptoms is unclear. Here we sought to address this issue by studying mice homozygous for the R200W Vhl mutation (VhlR/R mice) as a model for Chuvash disease. These mice developed pulmonary hypertension independently of polycythemia and enhanced normoxic respiration similar to Chuvash patients, further validating VhlR/R mice as a model for Chuvash disease. Lungs from VhlR/R mice exhibited pulmonary vascular remodeling, hemorrhage, edema, and macrophage infiltration, and lungs from older mice also exhibited fibrosis. HIF-2alpha activity was increased in lungs from VhlR/R mice, and heterozygosity for Hif2a, but not Hif1a, genetically suppressed both the polycythemia and pulmonary hypertension in the VhlR/R mice. Furthermore, Hif2a heterozygosity resulted in partial protection against vascular remodeling, hemorrhage, and edema, but not inflammation, in VhlR/R lungs, suggesting a selective role for HIF-2alpha in the pulmonary pathology and thereby providing insight into the mechanisms underlying pulmonary hypertension. These findings strongly support a dependency of the Chuvash phenotype on HIF-2alpha and suggest potential treatments for Chuvash patients.
Figures

Similar articles
-
von Hippel-Lindau mutation in mice recapitulates Chuvash polycythemia via hypoxia-inducible factor-2alpha signaling and splenic erythropoiesis.J Clin Invest. 2007 Dec;117(12):3879-89. doi: 10.1172/JCI32614. J Clin Invest. 2007. PMID: 17992257 Free PMC article.
-
Therapeutic inhibition of HIF-2α reverses polycythemia and pulmonary hypertension in murine models of human diseases.Blood. 2021 May 6;137(18):2509-2519. doi: 10.1182/blood.2020009138. Blood. 2021. PMID: 33512384 Free PMC article.
-
Decreased serum glucose and glycosylated hemoglobin levels in patients with Chuvash polycythemia: a role for HIF in glucose metabolism.J Mol Med (Berl). 2013 Jan;91(1):59-67. doi: 10.1007/s00109-012-0961-5. Epub 2012 Sep 27. J Mol Med (Berl). 2013. PMID: 23015148 Free PMC article.
-
Involvement of oxygen-sensing pathways in physiologic and pathologic erythropoiesis.Blood. 2009 Sep 3;114(10):2015-9. doi: 10.1182/blood-2009-05-189985. Epub 2009 Jun 3. Blood. 2009. PMID: 19494350 Review.
-
Heritable disorders of oxygen sensing.Am J Med Genet A. 2021 Nov;185(11):3334-3339. doi: 10.1002/ajmg.a.62521. Am J Med Genet A. 2021. PMID: 34655169 Review.
Cited by
-
High-Altitude Andean H194R HIF2A Allele Is a Hypomorphic Allele.Mol Biol Evol. 2023 Jul 5;40(7):msad162. doi: 10.1093/molbev/msad162. Mol Biol Evol. 2023. PMID: 37463421 Free PMC article.
-
Regulation of hypoxia-induced pulmonary hypertension by vascular smooth muscle hypoxia-inducible factor-1α.Am J Respir Crit Care Med. 2014 Feb 1;189(3):314-24. doi: 10.1164/rccm.201302-0302OC. Am J Respir Crit Care Med. 2014. PMID: 24251580 Free PMC article.
-
High levels of zinc-protoporphyrin identify iron metabolic abnormalities in pulmonary arterial hypertension.Clin Transl Sci. 2011 Aug;4(4):253-8. doi: 10.1111/j.1752-8062.2011.00301.x. Clin Transl Sci. 2011. PMID: 21884511 Free PMC article.
-
Regulation of ventilatory sensitivity and carotid body proliferation in hypoxia by the PHD2/HIF-2 pathway.J Physiol. 2016 Mar 1;594(5):1179-95. doi: 10.1113/JP271050. Epub 2015 Oct 6. J Physiol. 2016. PMID: 26337139 Free PMC article.
-
Prolyl-4 Hydroxylase 2 (PHD2) Deficiency in Endothelial Cells and Hematopoietic Cells Induces Obliterative Vascular Remodeling and Severe Pulmonary Arterial Hypertension in Mice and Humans Through Hypoxia-Inducible Factor-2α.Circulation. 2016 Jun 14;133(24):2447-58. doi: 10.1161/CIRCULATIONAHA.116.021494. Epub 2016 Apr 25. Circulation. 2016. PMID: 27143681 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

