A comprehensive evaluation of the accuracy of cervical pre-cancer detection methods in a high-risk area in East Congo
- PMID: 20197765
- PMCID: PMC2844041
- DOI: 10.1038/sj.bjc.6605594
A comprehensive evaluation of the accuracy of cervical pre-cancer detection methods in a high-risk area in East Congo
Abstract
Background: Given the high burden of cervical cancer in low-income settings, there is a need for a convenient and affordable method for detecting and treating pre-cancerous lesions.
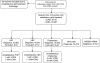
Methods: Samples for comparing the accuracy of cytology, virology and histology were collected. Identification of HPV E6/E7 mRNA was performed using PreTect HPV-Proofer. HPV DNA detection was performed by GP5+/6+ PCR, followed by reverse line blot (RLB) for typing.
Results: A total of 343 women, aged 25-60 years, attending gynaecological polyclinics in DR Congo were included for sample enrolment. The test positivity rate was conventional and liquid-based cytology (LBC) at cutoff ASCUS+ of 6.9 and 6.6%, respectively; PreTect HPV-Proofer of 7.3%; and consensus DNA PCR for 14 HR types of 18.5%. Sixteen cases of CIN2+ lesions were identified. Of these, conventional cytology identified 66.7% with a specificity of 96.2%, LBC identified 73.3% with a specificity of 96.9%, all at cutoff ASCUS+. HR-HPV DNA detected all CIN2+ cases with a specificity of 85.9%, whereas PreTect HPV-Proofer gave a sensitivity of 81.3% and a specificity of 96.6%.
Conclusion: Both HPV detection assays showed a higher sensitivity for CIN2+ than did cytological methods. Detecting E6/E7 mRNA from only a subset of HR HPVs, as is the case with PreTect HPV-Proofer, resulted in a similar specificity to cytology and a significantly higher specificity than consensus HR HPV DNA (P<0.0001).
Figures


References
-
- Arbyn M, Hovland S (2006) Test characteristics of multiple cytological and HPV nucleic acid assays to detect high-grade CIN. A study on 343 women from South-Kivu (Democratic Republic of Congo). IPH/EPI-REPORTS 1: 1–60. Brussels
-
- Arbyn M, Sankaranarayanan R, Muwonge R, Keita N, Dolo A, Mbalawa CG, Nouhou H, Sakande B, Wesley R, Somanathan T, Sharma A, Shastri S, Basu P (2008) Pooled analysis of the accuracy of five cervical cancer screening tests assessed in eleven studies in Africa and India. Int J Cancer 123: 153–160 - PubMed
-
- Baier T, Hansen-Hagge TE, Gransee R, Crombe A, Schmahl S, Paulus C, Drese KS, Keegan H, Martin C, O’Leary JJ, Furuberg L, Solli L, Grønn P, Falang IM, Karlgård A, Gulliksen A, Karlsen F (2009) Hands-free sample preparation platform for nucleic acid analysis. Lab on a Chip 9: 3399–3405 - PubMed
-
- Bosch FX, de Sanjose S (2003) Chapter 1: human papillomavirus and cervical cancer-burden and assessment of causality. J Natl Cancer Inst Monogr 2003(31): 3–13. Review - PubMed

