The G-protein-coupled formylpeptide receptor FPR confers a more invasive phenotype on human glioblastoma cells
- PMID: 20197768
- PMCID: PMC2844039
- DOI: 10.1038/sj.bjc.6605591
The G-protein-coupled formylpeptide receptor FPR confers a more invasive phenotype on human glioblastoma cells
Abstract
Background: The G-protein-coupled formylpeptide receptor (FPR) that mediates chemotaxis of phagocytic leucocytes induced by bacterial and host-derived chemotactic peptides is selectively expressed by highly malignant human gliomas and contributes to tumour growth and angiogenesis. As invasion of surrounding normal tissues is one of the important features of tumour malignancy, we investigated the function of FPR in the invasive behaviour of human glioblastoma cells.
Methods: Cells (FPR(+) and FPR(-)) were isolated by single-cell cloning from a human glioblastoma cell line U-87MG. The FPR expression was assayed by flow cytometry and reverse transcription PCR. The function of FPR was investigated by chemotaxis and calcium flux induced by FPR agonist fMLF. Tumour cell motility was assayed by a wound-healing model in vitro. The growth and invasive phenotype were observed with subcutaneous implantation of tumour cells in nude mice. Over-expression of FPR in FPR(-) cells was performed by transfection of a plasmid vector-containing human FPR gene.
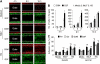
Results: One of the glioma clones F9 that expressed high level of FPR showed a more 'motile' phenotype in vitro as compared with a clone G3 without FPR expression. Although F9 and G3 clones both formed subcutaneous tumours in nude mice, only F9 tumours invaded surrounding mouse connective tissues. Over-expression of FPR in G3 clone (G3F) increased the cell motility in vitro and the capacity of the cells to form more rapidly growing and invasive tumours in nude mice. We further found that, in addition to supernatant of necrotic tumour cells, foetal calf serum and human serum used in culture media contained FPR agonist activity and increased the motility of FPR-expressing glioblastoma cells.
Conclusion: The expression of FPR is responsible for increased motility of human glioblastoma cells and their formation of highly invasive tumours.
Figures




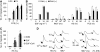
References
-
- Babbin BA, Lee WY, Parkos CA, Winfree LM, Akyildiz A, Perretti M, Nusrat A (2006) Annexin I regulates SKCO-15 cell invasion by signaling through formyl peptide receptors. J Biol Chem 281(28): 19588–19599 - PubMed
-
- Cheng N, He R, Tian J, Dinauer MC, Ye RD (2007) A critical role of protein kinase C delta activation loop phosphorylation in formyl-methionyl-leucyl-phenylalanine-induced phosphorylation of p47(phox) and rapid activation of nicotinamide adenine dinucleotide phosphate oxidase. J Immunol 179(11): 7720–7728 - PubMed
-
- Chintala SK, Sawaya R, Aggarwal BB, Majumder S, Giri DK, Kyritsis AP, Gokaslan ZL, Rao JS (1998) Induction of matrix metalloproteinase-9 requires a polymerized actin cytoskeleton in human malignant glioma cells. J Biol Chem 273(22): 13545–13551 - PubMed
-
- De Paulis A, Ciccarelli A, de Crescenzo G, Cirillo R, Patella V, Marone G (1996) Cyclosporin H is a potent and selective competitive antagonist of human basophil activation by N-formyl-methionyl-leucyl-phenylalanine. J Allergy Clin Immunol 98(1): 152–164 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

