The neuropathology of autism: defects of neurogenesis and neuronal migration, and dysplastic changes
- PMID: 20198484
- PMCID: PMC2869041
- DOI: 10.1007/s00401-010-0655-4
The neuropathology of autism: defects of neurogenesis and neuronal migration, and dysplastic changes
Abstract
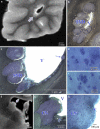
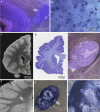
Autism is characterized by a broad spectrum of clinical manifestations including qualitative impairments in social interactions and communication, and repetitive and stereotyped patterns of behavior. Abnormal acceleration of brain growth in early childhood, signs of slower growth of neurons, and minicolumn developmental abnormalities suggest multiregional alterations. The aim of this study was to detect the patterns of focal qualitative developmental defects and to identify brain regions that are prone to developmental alterations in autism. Formalin-fixed brain hemispheres of 13 autistic (4-60 years of age) and 14 age-matched control subjects were embedded in celloidin and cut into 200-mum-thick coronal sections, which were stained with cresyl violet and used for neuropathological evaluation. Thickening of the subependymal cell layer in two brains and subependymal nodular dysplasia in one brain is indicative of active neurogenesis in two autistic children. Subcortical, periventricular, hippocampal and cerebellar heterotopias detected in the brains of four autistic subjects (31%) reflect abnormal neuronal migration. Multifocal cerebral dysplasia resulted in local distortion of the cytoarchitecture of the neocortex in four brains (31%), of the entorhinal cortex in two brains (15%), of the cornu Ammonis in four brains and of the dentate gyrus in two brains. Cerebellar flocculonodular dysplasia detected in six subjects (46%), focal dysplasia in the vermis in one case, and hypoplasia in one subject indicate local failure of cerebellar development in 62% of autistic subjects. Detection of flocculonodular dysplasia in only one control subject and of a broad spectrum of focal qualitative neuropathological developmental changes in 12 of 13 examined brains of autistic subjects (92%) reflects multiregional dysregulation of neurogenesis, neuronal migration and maturation in autism, which may contribute to the heterogeneity of the clinical phenotype.
Figures


References
-
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000.
-
- Andrade DM. Genetic basis in epilepsies caused by malformations of cortical development and in those with structurally normal brain. Hum Genet. 2009;126:173–193. - PubMed
-
- Avila J, Dominguez J, Diaz-Nido J. Regulation of microtubule dynamics by microtubule-associated protein expression and phosphorylation during neuronal development. Int J Dev Biol. 1994;38:13–25. - PubMed
-
- Bailey AP, Luthert A, Dean B, et al. A clinicopathological study of autism. Brain. 1998;121:889–905. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

