A dominant-negative needle mutant blocks type III secretion of early but not late substrates in Yersinia
- PMID: 20199604
- PMCID: PMC2911021
- DOI: 10.1111/j.1365-2958.2010.07096.x
A dominant-negative needle mutant blocks type III secretion of early but not late substrates in Yersinia
Abstract
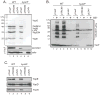
Yersinia pseudotuberculosis uses a type III secretion system (T3SS) to deliver effectors into host cells. A key component of the T3SS is the needle, which is a hollow tube on the bacterial surface through which effectors are secreted, composed of the YscF protein. To study needle assembly, we performed a screen for dominant-negative yscF alleles that prevented effector secretion in the presence of wild-type (WT) YscF. One allele, yscF-L54V, prevents WT YscF secretion and needle assembly, although purified YscF-L54V polymerizes in vitro. YscF-L54V binds to its chaperones YscE and YscG, and the YscF-L54V-EG complex targets to the T3SS ATPase, YscN. We propose that YscF-L54V stalls at a binding site in the needle assembly pathway following its release from the chaperones, which blocks the secretion of WT YscF and other early substrates required for building a needle. Interestingly, YscF-L54V does not affect the activity of pre-assembled actively secreting machines, indicating that a factor and/or binding site required for YscF secretion is absent from T3SS machines already engaged in effector secretion. Thus, substrate switching may involve the removal of an early substrate-specific binding site as a mechanism to exclude early substrates from Yop-secreting machines.
Figures






Similar articles
-
Structural characterization of the Yersinia pestis type III secretion system needle protein YscF in complex with its heterodimeric chaperone YscE/YscG.J Mol Biol. 2008 Mar 28;377(3):819-30. doi: 10.1016/j.jmb.2007.12.067. Epub 2008 Jan 5. J Mol Biol. 2008. PMID: 18281060 Free PMC article.
-
The YscE/YscG chaperone and YscF N-terminal sequences target YscF to the Yersinia pestis type III secretion apparatus.Microbiology (Reading). 2018 Mar;164(3):338-348. doi: 10.1099/mic.0.000610. Epub 2018 Feb 5. Microbiology (Reading). 2018. PMID: 29458689 Free PMC article.
-
Mutations in the Yersinia pseudotuberculosis type III secretion system needle protein, YscF, that specifically abrogate effector translocation into host cells.J Bacteriol. 2007 Jan;189(1):83-97. doi: 10.1128/JB.01396-06. Epub 2006 Oct 27. J Bacteriol. 2007. PMID: 17071752 Free PMC article.
-
Regulation of the Yersinia type III secretion system: traffic control.Front Cell Infect Microbiol. 2013 Feb 6;3:4. doi: 10.3389/fcimb.2013.00004. eCollection 2013. Front Cell Infect Microbiol. 2013. PMID: 23390616 Free PMC article. Review.
-
Links between type III secretion and extracytoplasmic stress responses in Yersinia.Front Cell Infect Microbiol. 2012 Oct 10;2:125. doi: 10.3389/fcimb.2012.00125. eCollection 2012. Front Cell Infect Microbiol. 2012. PMID: 23087910 Free PMC article. Review.
Cited by
-
Protein export according to schedule: architecture, assembly, and regulation of type III secretion systems from plant- and animal-pathogenic bacteria.Microbiol Mol Biol Rev. 2012 Jun;76(2):262-310. doi: 10.1128/MMBR.05017-11. Microbiol Mol Biol Rev. 2012. PMID: 22688814 Free PMC article. Review.
-
DsbA2 (27 kDa Com1-like protein) of Legionella pneumophila catalyses extracytoplasmic disulphide-bond formation in proteins including the Dot/Icm type IV secretion system.Mol Microbiol. 2011 May;80(3):835-52. doi: 10.1111/j.1365-2958.2011.07615.x. Epub 2011 Mar 22. Mol Microbiol. 2011. PMID: 21375592 Free PMC article.
-
Surface organelles assembled by secretion systems of Gram-negative bacteria: diversity in structure and function.FEMS Microbiol Rev. 2012 Nov;36(6):1046-82. doi: 10.1111/j.1574-6976.2012.00342.x. Epub 2012 May 24. FEMS Microbiol Rev. 2012. PMID: 22545799 Free PMC article. Review.
-
A C-terminal region of Yersinia pestis YscD binds the outer membrane secretin YscC.J Bacteriol. 2011 May;193(9):2276-89. doi: 10.1128/JB.01137-10. Epub 2011 Feb 25. J Bacteriol. 2011. PMID: 21357482 Free PMC article.
-
Kinase Activities of RIPK1 and RIPK3 Can Direct IFN-β Synthesis Induced by Lipopolysaccharide.J Immunol. 2017 Jun 1;198(11):4435-4447. doi: 10.4049/jimmunol.1601717. Epub 2017 May 1. J Immunol. 2017. PMID: 28461567 Free PMC article.
References
-
- Abe A, de Grado M, Pfuetzner RA, Sanchez-Sanmartin C, Devinney R, Puente JL, Strynadka NC, Finlay BB. Enteropathogenic Escherichia coli translocated intimin receptor, Tir, requires a specific chaperone for stable secretion. Mol Microbiol. 1999;33:1162–1175. - PubMed
-
- Agrain C, Sorg I, Paroz C, Cornelis GR. Secretion of YscP from Yersinia enterocolitica is essential to control the length of the injectisome needle but not to change the type III secretion substrate specificity. Mol Microbiol. 2005;57:1415–1427. - PubMed
-
- Akeda Y, Galan JE. Chaperone release and unfolding of substrates in type III secretion. Nature. 2005;437:911–915. - PubMed
-
- Allaoui A, Schulte R, Cornelis GR. Mutational analysis of the Yersinia enterocolitica virC operon: characterization of yscE, F, G, I, J, K required for Yop secretion and yscH encoding YopR. Mol Microbiol. 1995;18:343–355. - PubMed
-
- Amann E, Ochs B, Abel KJ. Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene. 1988;69:301–315. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

